This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “UV – Visible Spectroscopy”.
1. What is the wavelength range for UV spectrum of light?
a) 400 nm – 700 nm
b) 700 nm to 1 mm
c) 0.01 nm to 10 nm
d) 10 nm to 400 nm
View Answer
Explanation: Ultraviolet (UV) is an electromagnetic radiation with a wavelength from 10 nm to 400 nm, shorter than that of visible light but longer than X-rays (the visible region fall between 380-750 nm and X- rays region fall between 0.01 to 10nm).
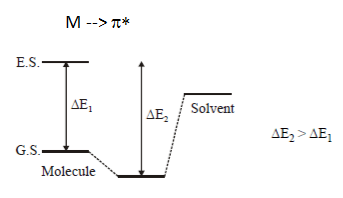
2. Which of the following comparison is correct for solvent shift on the n –>π* transition of acetone?
a) H20 = CH30H = C2H50H = CHC13 = C6H14
b) H20 > CH30H > C2H50H > CHC13 > C6H14
c) H20 < CH30H < C2H50H < CHC13 < C6H14
d) H20 > CH30H < C2H50H < CHC13 < C6H14
View Answer
Explanation: H-bonding with ground state in n–> π* results in increase in energy gap & decrease in wavelength.
And as polar solvents show strong H-bonding. So, the correct option is H20 < CH30H < C2H50H < CHC13 < C6H14.
3. What is the correct order of λmax for n –> σ* transition?
a) R-OH > R-NH2 > R-SH
b) R-OH < R-NH2 < R-SH
c) R-OH > R-SH > R-NH2
d) R-OH < R-SH < R-NH2
View Answer
Explanation: According to molecular orbital energy diagram for R-OH, R-NH2, R-SH (shown below), energy level will decrease respectively so λmax will increase.
4. What is the correct order of λmax for n –> π* transition for the R-CN, R-NO2, and R-N=N-R?
a) R-CN < R-NO2 < R-N=N-R
b) R-CN = R-NO2 = R-N=N-R
c) R-CN > R-NO2 > R-N=N-R
d) R-CN > R-NO2 < R-N=N-R
View Answer
Youtube | Telegram | LinkedIn | Instagram | Facebook | Twitter | Pinterest


Subscribe to his free Masterclasses at Youtube & discussions at Telegram SanfoundryClasses.