This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparations of Glycerol”.
1. Glycerol can be formed through digestion of which of the following?
a) galactose
b) fats
c) glucose
d) sucrose
View Answer
Explanation: Heating fats(triglyceride) will leads to formation of glycerol along with formation of corresponding acid.
2. What is the name of the process of formation of glycerol via formation of allyl chloride?
a) Epichlorohydrine
b) Acrolein
c) Propylene oxide
d) Chloroform process
View Answer
Explanation: The epichlorohydrin process is the most important; it involves the chlorination of propylene to give allyl chloride, which is oxidized with hypochlorite to dichlorohydrins, which reacts with a strong base to give epichlorohydrin. This epichlorohydrin is then hydrolyzed to give glycerol.
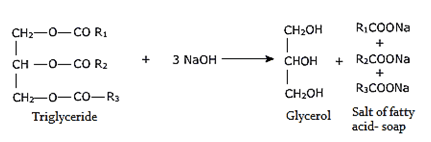
3. Fatty acids and glycerol (C3H8O3) are produced after hydrolysis of which of the following?
a) amino acids
b) fats
c) starch
d) cellulose
View Answer
Explanation: On hydrolysis in presence of an alkali, the tri esters yield glycerol and the fall of the carboxylic acids.
4. What is the middle product ‘A’ in the formation of the glycerol by propylene?
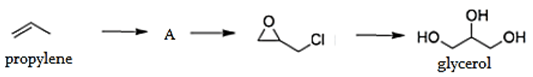
a) Allyl chloride
b) Vinyl chloride
c) Acyl chloride
d) Dichloroalkane
View Answer
Explanation: The epichlorohydrin process is the most important; it involves the chlorination of propylene to give allyl chloride, which is oxidized with hypochlorite to dichlorohydrins, which reacts with a strong base to give epichlorohydrin. This epichlorohydrin is then hydrolyzed to give glycerol.
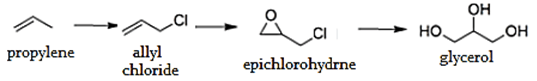
5. What is the product A formed by the partial oxidation using metal oxides and air of propylene or the given preparation of the glycerol?
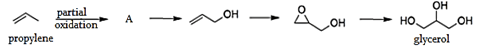
a) Propene
b) Acroline
c) Propylene oxide
d) Propanol
View Answer
Explanation: Processes from propylene include the synthesis of glycerol from acrolein by partial oxidation of propylene. Acrolein further form propen-3-ol and then hydrolyzed to give glycerol.
6. What is the product A formed by the partial oxidation using tert- butyl hydroperoxides or hydrogen peroxide of propylene or the given preparation of the glycerol?
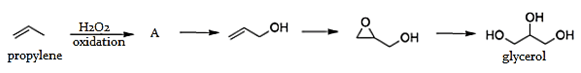
a) Propene
b) Acroline
c) Propylene oxide
d) Propanol
View Answer
Explanation: Processes from propylene include the synthesis of glycerol from propylene oxide is from oxidation by H2O2 or tert- butyl hydroperoxides of propylene. Propylene oxide further form propen-3-ol and then hydrolyzed to give glycerol.
7. Why synthetic production of glycerol is not commercially successful?
a) Because process is expensive
b) Because no marketing demands
c) Because process is hazardous
d) Because of the large-scale production of biodiesel from fats
View Answer
Explanation: Because of the large-scale production of biodiesel from fats, where glycerol is a waste product, the market for glycerol is depressed. Thus, synthetic processes are not economical. Owing to oversupply, efforts are being made to convert glycerol to synthetic precursors, such as acrolein.
8. Which of the following is not the step for the isolation of glycerine form spent lye?
a) Brine Solution Preparation
b) Saponification and salting
c) Zone distillation
d) Glycerin Recovery from Spent Soap Lye
View Answer
Explanation: Here zone distillation is not possible as constituents are in liquid phase. Glycerol is a high boiling liquid with boiling point 290℃. Here distillation will be done under high pressure at different temperature.
9. What will be the product X in the formation of glycerol?
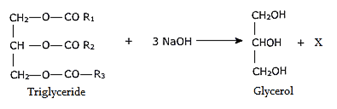
a) R-ONa
b) RCOH
c) RCOONa
d) R-ONa and RCOONa both can be formed
View Answer
Explanation: On hydrolysis in presence of an alkali, the tri esters yield glycerol and the fall of the carboxylic acids.
10. What will be the reagent and conditions required for the given reaction of preparation of glycerol form propene from given conditions respectively?
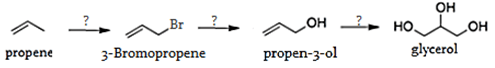
a) NBS, NaOH/OH–, OsO4
b) NaOH/OH–, NBS, alk. MnO4–
c) alk. MnO4–, NaOH/OH–, NBS
d) NBS, NaOH/OH–, OsO4
View Answer
Explanation: Firstly, propene will be brominated by N-bromo succinimide (NBS), then substitution reaction will take place by OH– via SN2 mechanism and finally then addition of hydoxy group on propen-3-ol by OsO4 or alkaline KMnO4, will take place to give glycerol.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
