This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Physical Properties of Aldehydes”.
1. Aldehydes have which type of smell?
a) Fish like smell
b) Bitter almond smell
c) Pungent smell
d) Rotten egg like smell
View Answer
Explanation: The volatile aldehydes have pungent odors. benzaldehyde have bitter almond type smell. Aldehydes have Pungent type smell.
2. What is the name of the process in which aldehyde get oxidise in presence of air?
a) Calcination
b) Autoxidation
c) Cannizzaro reaction
d) Baeyer villiger oxidation
View Answer
Explanation: Autoxidation is oxidation that occurs in open air or in presence of oxygen (and sometimes UV radiation) and forms peroxides and hydroperoxides. It can be considered to be a slow, flameless combustion of materials by reaction with oxygen. Autoxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives, and also because it occurs in situations where it is not desired.
3. Which of the following aldehyde shows oligomerization?
a) Acetaldehyde
b) Propanal
c) Butanal
d) Benzaldehyde
View Answer
Explanation: The two aldehydes of greatest importance in industry, formaldehyde and acetaldehyde, have complicated behavior because of their tendency to oligomerize or polymerize.
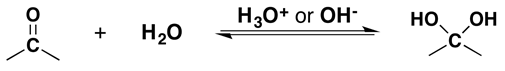
4. What will be the product if we add water to the aldehyde?
a) Alcohols
b) Epoxides
c) Geminal diols
d) Peroxides
View Answer
Explanation: It has been demonstrated that water, in the presence of an acid or a base, adds rapidly to the carbonyl function of aldehydes and ketones establishing a reversible equilibrium with a geminal-diol or gem-diol.
5. In Infrared spectroscopy, at what wave number will band of CO bond of aldehyde will occur?
a) 1500 cm-1
b) 1495 cm-1
c) 1965 cm-1
d) 1700 cm-1
View Answer
Explanation: Using IR spectroscopy, they display a strong CO band of aldehyde will occur near to 1700 cm-1.
6. What is the chemical shift of formyl hydrogen in aldehyde?
a) 6
b) 7
c) 8
d) 9
View Answer
Explanation: In their 1H NMR spectra, the formyl hydrogen center absorbs near δH = 9, which is a distinctive part of the spectrum. This signal shows the characteristic coupling to any protons on the alpha carbon.
7. Which of the following aldehyde is present as gas?
a) Acetaldehyde
b) Formaldehyde
c) Butyraldehyde
d) Benzaldehyde
View Answer
Explanation: Methanal is a gas (boiling point -21°C), and ethanal has a boiling point of +21°C. That means that ethanal boils at close to room temperature. The other aldehydes and the ketones are liquids, with boiling points rising as the molecules get bigger.
8. Which of the following compound has more boiling point than aldehyde?
a) Alcohol
b) Alkanes
c) Ketones
d) Ether
View Answer
Explanation: The aldehyde (with dipole-dipole attractions as well as dispersion forces) has a boiling point higher than the similarly sized alkane which only has dispersion forces and because of more possibility of Hydrogen Bonding and (resonance after so) thus increasing linkage and weight of molecules making it difficult for boiling off, so aldehyde will have higher boiling point than ether and ketones. However, the aldehyde’s boiling point isn’t as high as the alcohol’s. In the alcohol, there is hydrogen bonding as well as the other two kinds of intermolecular attraction.
9. Which of the following is the characteristic smell of benzaldehyde?
a) Fish like smell
b) Bitter almond like
c) Pungent smell
d) Rotten egg like smell
View Answer
Explanation: It is a colorless liquid with a characteristic almond-like odor. The primary component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources.
10. Which of the following aldehyde is most soluble in water?
a) Acetaldehyde
b) Formaldehyde
c) Butyraldehyde
d) Benzaldehyde
View Answer
Explanation: As the carbon chain increases in length, solubility in water decreases.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
