This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Five Membered Rings”.
1. Which of the following is not true about the five membered rings?
a) Five membered rings are more stable than 4 membered rings
b) Five membered rings are more stable than 6 membered rings
c) Five membered rings are more stable than 7 membered rings
d) Five membered rings are more stable than 8 membered rings
View Answer
Explanation: 6-membered rings can have 3D conformations, such as the chair conformation (the more stable) and the boat conformation. These conformations relax the angles, getting them closer to the tetrahedral angle. Thus, cycle gains stability. That is why 5-membered rings are less stable than 6-membered.
2. Which of the following is a not a five membered ring?
a) Pyridine
b) Pyrrole
c) Furan
d) Thiophene
View Answer
Explanation: Pyridine is a basic five membered heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with on (=CH–) group replaced by a nitrogen atom.
3. Which of the following five membered rings is most resonance stabilized?
a) Furan
b) Thiophene
c) Pyrrole
d) Pyridine
View Answer
Explanation: Thiophene is most resonance stabilized five membered rings among above compounds. As thiophene has Sulphur and least electronegativity ring than nitrogen and oxygen in pyrrole and furan respectively.
4. Five membered rings come under which category of heterocycle classification on the basis of chemical behavior?
a) -excessive heterocycle
b) -deficient heterocycle
c) -equivalent heterocycle
d) Can’t say about the five membered rings
View Answer
Explanation: In five membered ring, 6 π electrons are distributed between 5 atoms, each atom shares more than 1 e– (1.2e–).
5. What is the reactivity order in the following five membered heterocyclic compounds?
a) Pyrrole
b) Furan
c) Thiophene
d) Pyridine
View Answer
Explanation: Pyrrole is more reactive than furan and thiophene in electrophilic reactions. Therefore; pyrrole is more prone to electrophilic substitution than furan. The nitrogen atom in pyrrole can conjugate with the π-electrons on the ring, so the density of the π-electrons on the ring will increase.
6. What is the product when thiophene reacts with Br2 in benzene?
a) 2-bromothiophene
b) 3-bromothiophene
c) 2,5-dibromothiophene
d) 3,4-dibromothiophene
View Answer
Explanation: This is electrophilic substitution reaction, bromination of thiophene in presence of benzene leads to formation of 2,5-dibromothiophene.

7. What is the product when pyrrole reacts with Br2 in ethanol?
a) 2,3-dibromopyrrole
b) 2,3,4,5-tetrabromopyrrole
c) 2,5-dibromopyrrole
d) 3,4-dibromopyrrole
View Answer
Explanation: This is electrophilic substitution reaction, bromination of pyrrole in presence of ethanol leads to formation of 2,3,4,5-tetrabromopyrrole.
8. What is the name of the following reaction?
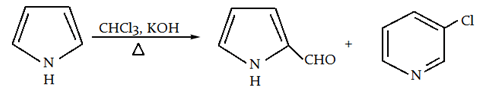
a) Gattermann reaction
b) Riemer tiemann reaction
c) Friedal craft reaction
d) Blanc’s chloromethylation
View Answer
Explanation: This is a Riemer tiemann reaction, in which boiling pyrrole will react with aqueous or alcoholic potash and chloroform.
9. What is the name of the following reaction?
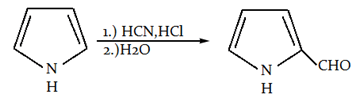
a) Gattermann reaction
b) Riemer tiemann reaction
c) Friedal craft reaction
d) Blanc’s chloromethylation
View Answer
Explanation: This is a Gattermann reaction, in which boiling pyrrole will react with HCN and HCl followed by addition of water and leads to formation of pyrole-2-carbaldehyde.
10. What will be the reagent used for the completion of the following reaction?
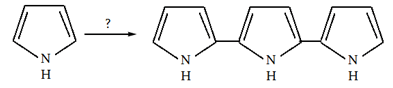
a) Concentrated acid
b) Dilute acid
c) Concentrated base
d) Dilute base
View Answer
Explanation: In dilute acid for a brief time, pyrrole form trimer.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
