This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Nucleophilic Substitution in Pyridine”.
1. At which position of pyridine nucleophilic substitution reaction is most preferred?
a) First and third
b) Second
c) Third
d) Second and Forth
View Answer
Explanation: When the nucleophile is at the 2 and 4 positions, the intermediate anion is stabilized by the electronegative nitrogen atom. This results in a more stable anion as compared to the intermediate anion when the nucleophile is at the 3 positions. Hence, the 2 and 4 positions is preferred for nucleophilic substitution.
2. Pyridine undergoes nucleophilic substitution with NaNH2 at 100℃ to give which of the following?
a) 3-aminopyridine
b) 2-aminopyridine
c) 3,5-diaminopyridine
d) 2,5-diaminopyridine
View Answer
Explanation: Here, sodium amide is used as the nucleophile yielding 2-aminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
3. Pyridine undergoes nucleophilic substitution with excess of NaNH2 at 100℃ to give which of the following?
a) 3-aminopyridine
b) 2,6-diaminopyridine
c) 3,5-diaminopyridine
d) 2,5-diaminopyridine
View Answer
Explanation: Sodium amide in excess is used as the nucleophile yielding 2,6-diaminopyridine. The hydride ion released in this reaction combines with a proton of an available amino group, forming a hydrogen molecule.
4. What will be the product of the following reaction?
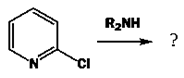
a) 3-aminopyridine
b) 2-aminopyridine
c) 3,5-diaminopyridine
d) 2,5-diaminopyridine
View Answer
Explanation: We can see the ease of replacement of halogens in these positions by nucleophiles. The intermediate anion is stabilized by electronegative nitrogen and by delocalization round the ring.
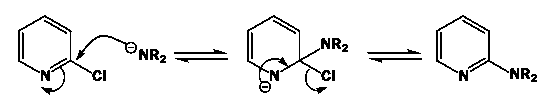
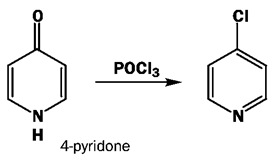
5. What will be the product of the following reaction?
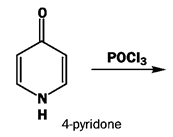
a) 3-chloropyridine
b) 2-dichloropyridine
c) 3,5-dichloropyridine
d) 2,5-dichloropyridine
View Answer
Explanation: This is the direct conversion to chloropyridines with POCl3. The reaction starts by attack of the oxygen atom at phosphorus to create a leaving group, followed by aromatic nucleophilic substitution.
6. Nucleophilic substitution reaction of pyridine is most effective in which of the conditions?
a) In slightly acidic conditions
b) In slightly basic conditions
c) In neutral medium
d) No specific condition requirements
View Answer
Explanation: The nitrogen atom makes pyridines more reactive towards nucleophilic substitution, particularly at the 2- and 4-positions, by lowering the LUMO energy of the π system of pyridine.
7. What is the name of the following reaction?
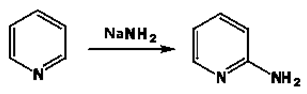
a) Chichibabin reaction
b) Dealkylation of pyridine
c) Hantzsch pyridine synthesis
d) Bonnemann cyclisation
View Answer
Explanation: The Chichibabin reaction is a method for producing 2-aminopyridinederivatives by the reaction of pyridine with sodium amide. The direct amination of pyridine with sodium amide takes place in liquid ammonia. Following the addition elimination mechanism first, a nucleophilic NH2− is added while a hydride (H−) is leaving.
8. Electron-withdrawing groups inhibit the Chichibabin reaction. Which of the following statement is not true about this fact?
a) they increase the basicity of the ring nitrogen
b) these electron-withdrawing groups can also form complexes with sodium amide
c) for single electron transfer pathway, altering the distribution of spin density of the intermediate radical anion
d) they slow down the sorption on sodium amide
View Answer
Explanation: Electron-withdrawing groups inhibit the Chichibabin reaction they decrease the basicity of the ring nitrogen by decreasing the density of electron on nitrogen. So the donation of an electron is less.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
