This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Grignard Reagent”.
1. Alkyl halides can be converted into Grignard reagents by _______________
a) Boiling them with Mg ribbon in alcoholic solution
b) Warming them with magnesium powder in dry ether
c) Refluxing them with MgCl2 solution
d) Warming them with Mgcl2
View Answer
Explanation: Alkyl halides can be converted into Grignard reagents by warming them with magnesium powder in dry ether.
RX + Mg + Dry ether → R−Mg−X
2. Which is not present in Grignard reagent?
a) Methyl group
b) Magnesium
c) Halogen
d) −COOH group
View Answer
Explanation: Grignard reagents are made from halogenoalkanes (haloalkanes or alkyl halides) and introduces some of their reactions. A Grignard reagent has a formula RMgX where X is a halogen, and R is an alkyl or aryl (based on a benzene ring) group.
3. Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide?
a) 3-methylpentanal
b) Ethyl benzoate
c) 4,4-dimethylcyclohexanone
d) 4-heptanone
View Answer
Explanation: 4-heptanone does not give a tertiary alcohol upon reaction with methylmagnesium bromide, as the Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively.
4. Which of the following statements about Grignard reagent is false?
a) Grignard reagents (RMgBr) add to the carbonyl group of aldehydes and ketones
b) An organosodium compound is not very reactive compared to a Grignard reagent
c) Grignard reagents are prepared in ether or tetrahydrofuran (THF)
d) Grignard reagents are decomposed by water and alcohol
View Answer
Explanation: It is supposed that the Mg-C bond is strongly polar covalent, not ionic. Grignard’s reagents are less reactive than organosodium, -potassium and -lithium compounds, that is the reason why it is more convenient to work with them.
5. Which of the following compounds gives a secondary alcohol upon reaction with methylmagnesium bromide?
a) Butyl formate
b) 3- pentanone
c) Pentanal
d) Methyl butanoate
View Answer
Explanation: Pentanal gives a secondary alcohol upon reaction with methylmagnesium bromide, as the Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively.
6. A Grignard’s reagent may be made by reacting magnesium with which of the following compound?
a) Methyl amine
b) Diethyl ether
c) Ethyl iodide
d) Ethyl alcohol
View Answer
Explanation: A Grignard’s reagent can be formed by reacting magnesium with Ethyl iodide, as shown below;
C2H5I + Mg + Dryether → C2H5 − Mg − I (Ethylmagnesium iodide)
7. Which of the following compounds gives a primary alcohol upon reaction with phenylmagnesium bromide?
a) 2-methyloxirane
b) ethylene oxide
c) ethyl formate
d) carbon dioxide
View Answer
Explanation: Ethylene oxide reacts with Grignard reagents to give, after protonation, primary alcohols with two additional carbon atoms.
8. Which of the following reagents, when treated with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol?
a) Ethanol
b) Diethyl ether
c) Ethanal
d) Oxirane
View Answer
Explanation: When is treated Diethylether with phenylmagnesiuim bromide followed by acid workup, will yield 2-phenylethanol.
Grignard reagents are made by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just “ether”). The flask is fitted with a reflux condenser, and the mixture is warmed over a water bath for 20 – 30 minutes.
CH3CH2Br + Mg + Diethyl ether → CH3CH2MgBr
Everything must be perfectly dry because Grignard reagents react with water.
9. Which of the following compounds would not give tert-butyl alcohol when treated with excess methylmagnesium bromide?
a) acetyl chloride
b) acetaldehyde
c) methyl acetate
d) acetic anhydride
View Answer
Explanation: Acetaldehyde would not give tert-butyl alcohol when treated with excess methylmagnesium bromide, as the Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively.
10. Which of the following reaction sequence that will best carry out the following preparation?
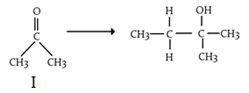
a) i I + MeONa + CH3H2Br ii) neutralize
b) i) I + EtONa ii) CH3CH2Br iii) neutralize
c) i) CH3CH2Br + Mg, Et2O ii) Add I iii) neutralize
d) i) I + CH3CH2OH + Mg ii) neutralize
View Answer
Explanation: In the first step, the Grignard forms the carbon-carbon bond. This results in an alkoxide (the conjugate base of an alcohol). To form the alcohol, it’s necessary to add acid at the end of the reaction and this reaction usually occurs in Et2O or THF followed by H3O+ work-ups.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
