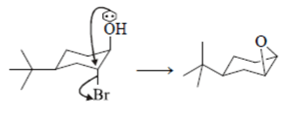
This set of Organic Chemistry test focuses on “Nucleophilic & Electrophilic Substitution Reaction”.
1. What is the correct statement for the given reactions?
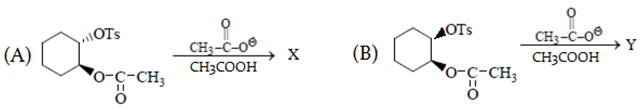
a) B reacts faster than A
b) Both give the same product
c) A gives trans and B gives c is product
d) A gives cis and B gives trans product
View Answer
Explanation:
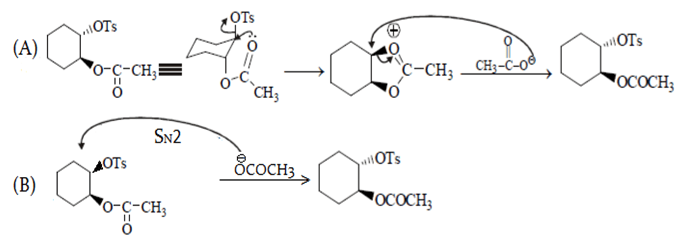
In reaction A, groups attached to benzene are on opposite plane so SNGP mechanism will be followed and B will undergo SN2. Due to SNGP (A) reacts faster than B and both give same product.
2. Which of the following statement is incorrect about nucleophiles?
a) Nucleophiles have an unshared electron pair and can make use of this to react with an electron deficient species
b) The nucleophilicity of an element (an electron donor) generally increases on going down a group in the periodic table
c) A nucleophile is electron-deficient species
d) All good nucleophiles are good bases when we deal across the period
View Answer
Explanation: A nucleophile is a chemical species that donates an electron pair to an electrophile to form a chemical bond in relation to a reaction, it is an electron rich species.
3. What is the correct order of nucleophilicity in the following options?
a) (CH3)3CO– > CH3–
b) CH3S– > CH3SH
c) CH3CH2CH2O– < (CH3)3CO–
d) (CH3CH2)3N > (CH3CH2)3P
View Answer
Explanation: Alkoxides are weaker Nu– than carbanion because negative charge on oxygen is more stable than carbon. CH3CH2CH2O– is more nucleophilic, due to less steric hindrance. The negative charge is more nucleophilic than a stable compound.
4. Which of the following order is correct for the solvolysis in 50% aqueous ethanol at 44.6oC?
a) 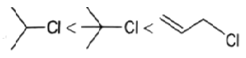
b) 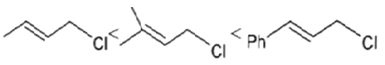
c) 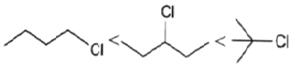
d) 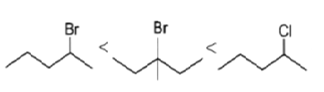
View Answer
Explanation: Solvolysis is a nucleophilic substitution (SN1) or elimination, where the nucleophile is a solvent molecule. Solvolysis order CH3X < 1o halide < 2o halide < allylic halide < 3o halide.
5. Identify correct step representing SN1 mechanism for the cleavage of ether with HI.
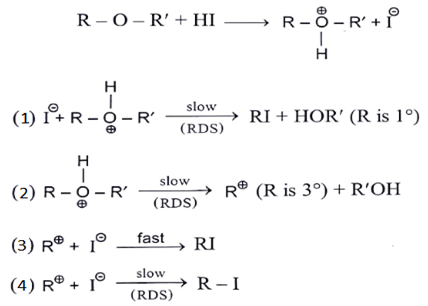
a) 1 and 3
b) 2 and 3
c) 1 and 4
d) 2 and 4
View Answer
Explanation: As we can see in below reaction first step is a slow step in which carbocation is formed. And the second step is fast where nucleophile will attach carbocation.
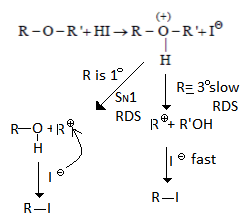
6. The relative rates of nucleophilic substitution for the given substrates are as follows:
| Compound | Approx. Relative rate |
|---|---|
| CH3CH2Br | 1.0 |
| CH3CH2CH2Br | 0.28 |
| (CH3)2CHCH2Br | 0.030 |
| (CH3)3 CCH2Br | 0.00000042 |
Which of the following statement is correct?
a) Each of the above reactions is likely to be SN2
b) Each of the above reactions is likely to be SN1
c) First two reactions follow SN2 and next two reactions follow SN1 pathway
d) There is no role of “steric factor”
View Answer
Explanation: As the steric hindrance increase, attack on carbon attached to Br decreases. It is possible only when reaction undergoes via SN2 reaction.
7. Which of the following reaction is not possible?
a) 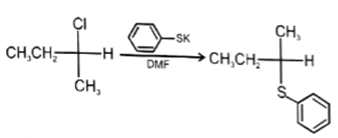
b)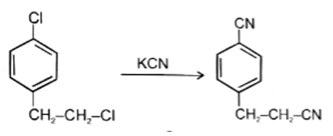
c) 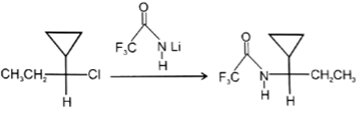
d)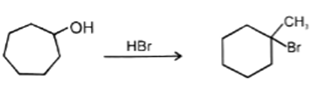
View Answer
Explanation: In reaction, the nucleophilic attack will be at aliphatic chlorine atom instead of aromatic chlorine because aromatic carbon SN2 is not possible.
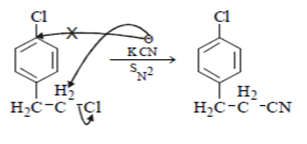
8. What will be the starting material (I) in the given reaction?
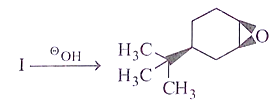
a) 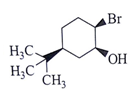
b)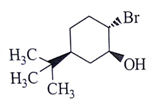
c)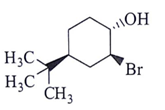
d)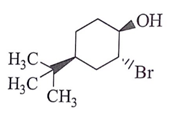
View Answer
9. Which of the following statement is true about the reaction given below?
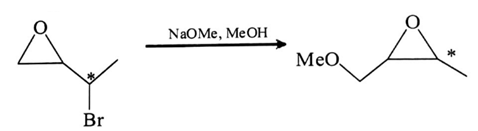
a) It involves a carbocation intermediate
b) The rearrangement is due to SN1 reaction mechanism
c) It proceeds via a concerted SN2 pathway
d) It proceeds via a concerted SN1 pathway
View Answer
Explanation: In this reaction nucleophile (–OMe) will attack at carbon attach to oxygen and in this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step, so it will undergo SN2 pathway.
10. Benzyl chloride is reacted with different nucleophiles (HO–, CH3COO–, PhO–, CH3O–). Arrange them in the decreasing order of reactivity with Benzyl chloride.
a) CH3O– > HO– > PhO– > CH3COO–
b) HO– > CH3O– > PhO– > CH3COO–
c) HO– > PhO– > CH3O– > CH3COO–
d) CH3COO– > CH3O– > HO– > PhO–
View Answer
Explanation: HO– > CH3O– > PhO– > CH3COO–
Here,CH3O– is most stable as the negative charge is distributed among two oxygen, followed by oxygen attach to phenyl group will share electron in resonance. And carbon is more electronegative than hydrogen attached to oxygen that’s why HO– is less stable than CH3O– and more nucleophilic.
11. Among the following compounds, what is the decreasing order of reactivity towards electrophilic substitution?
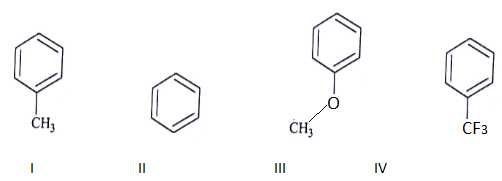
a) III > I > II > IV
b) IV > I > II > III
c) II > III > II > IV
d) I > III > II > IV
View Answer
Explanation: This is a electrophilic substitution and more the electrodensity on the ring faster the reaction.
III > I > II > IV (Reactivity order towards E substitution)
12. Among the following, which one is not a meta directing group in an electrophilic attack?
a)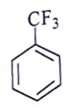
b) 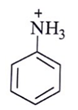
c) 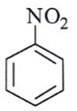
d) 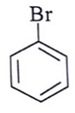
View Answer
Explanation: d id not a meta directing group in electrophilic attack because halogen are ortho- para directing and ring deactivationg group.
13. What will be the decreasing order of electrophilic nitration of the following compounds?
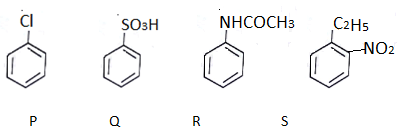
a) S > R > P > Q
b) R > S > P > Q
c) R > P > S > Q
d) P > S > R > Q
View Answer
Explanation: R > P > S > Q
In R electron donating group is present. In P, Cl will attract rings electron and donation will increase. In S there is electron donating group hence it is more reactive. If C2H5 is not there than this order reverses.
In Q electron pair will get into resonance.
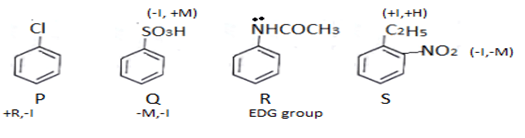
14. Nitro group is meta-directing in electrophilic aromatic substitution reactions?
a) increases electron density at meta-position
b) increases electrons density at ortho and para-positions
c) decreases electron density at meta-position
d) decreases electron density at ortho and para-positions
View Answer
Explanation: Because nitro group decreases electron density at ortho and para-positions, which will leads to deactivation of ring.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry for tests, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs