This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Nucleophilic Substitution Reaction”.
1. Which of the following compound shows the correct decreasing order of solvolysis with aqueous ethanol?
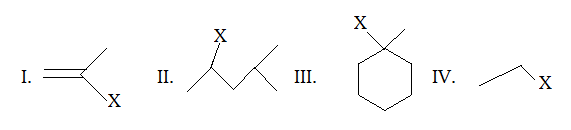
The correct choice is:
a) III > II > I > IV
b) III > II > IV > I
c) II > III > IV > I
d) III > I > IV > II
View Answer
Explanation: The solvation of above compound can be compared by this trend, i.e. Solvation trend: 3O halide > 2O halide > 1O halide > vinylic halide. So, (III) molecule is an example of 3O halide, (II) molecule is an example of 2O halide, (IV) molecule is an example of 1O halide and (I) molecule is an example of vinylic halide.
2. Which of the following reaction will go faster if the concentration of the nucleophile is raised?
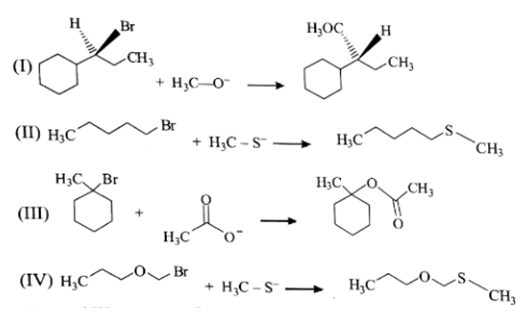
a) I and III
b) II and IV
c) I and II
d) I, II and IV
View Answer
Explanation: I & II reaction undergo via SN2 mechanism. III & IV reaction undergo via SN1 mechanism. As we know the rate of reaction in SN2 reaction is directly proportional to the concentration of nucleophile.
3. SN1 reaction undergoes through a carbocation intermediate as follows:
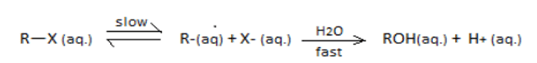
[R = t-Bu, iso-Pr, Et, Me] (X = Cl, Br, I)
The correct statements are:
I. The decreasing order of rate of SN1reaction is t-BuX > iso-PrX > EtX > MeX
II. The decreasing order of ionization energy is MeX > EtX > iso-PrX > t-BuX
III. The decreasing order of energy of activation is t-BuX > iso-PrX > EtX > MeX
a) I & II are correct
b) I & III are correct
c) II and III are correct
d) I, II & III are correct
View Answer
Explanation:
(1) Formation of carbocation is RDS, more stable the cation more easily it will form. Hence rate order is t-BuX > iso-PrX > EtX > MeX
(2) Formation of carbocation CH3 is most difficult hence ionization energy is highest. Similarly, EtX has lesser ionization energy than MeX and so on.
(3) Order of energy of activation is MeX > EtX > iso-PrX > t-BuX.
4. Which statement is incorrect about the following reaction?
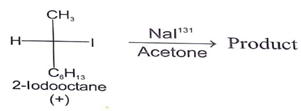
a) The rate of these reaction depends on both [R—I] and [131I-]
b) Loss of optical activity was twice as fast as the gain of radioactivity
c) Each molecule undergoing substitution suffers Inversion of configuration
d) The Final solution has radioactive iodine only
View Answer
Explanation:
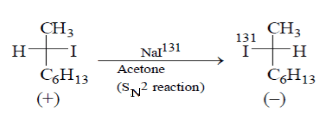
Here the rate of reaction is directly proportional to the concentration of RI and (131I-), so statement is correct.
If any molecule undergoes substitution, then it forms inversion product due to SN2 reaction. Hence statement is correct.
For statement, below reaction one (+) molecule converts into (–) molecular and it also converts one optical reaction of (+) molecule.
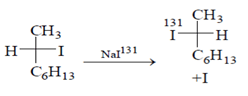
Final solution contains both Iodine (I131) and normal (I). So, we can say this is the only incorrect statement about the above reaction.
5. Which one is an excellent substrate for SN2 reaction?
a)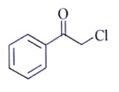
b)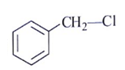
c) CH3 – CH2 – Cl
d)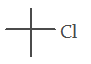
View Answer
Explanation: Alpha-halo carbonyl undergoes fastest SN2 reaction due to the coupling of π* of CO bond and π* of C–X bond.
6. Which of the following curve correctly represents SN1 vs SN2?
a)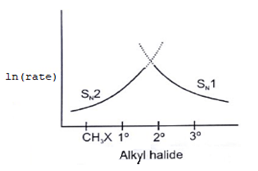
b)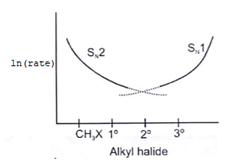
c)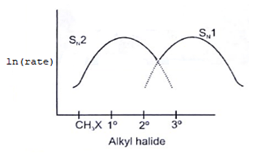
d)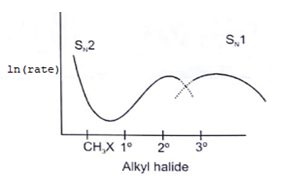
View Answer
Explanation: As we know rate order in SN2 is CH3X > 1o > 2o > 3o halide and rate order in SN1 is CH3X < 1o < 2o < 3o halide. So according to the following representation.
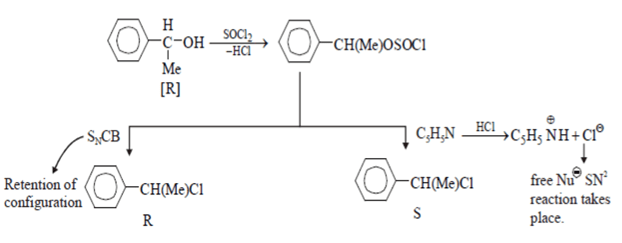
a) R, R
b) R, S
c) S, S
d) S, R
View Answer
Explanation: When ether reacts with the reactant nucleophilic substitution conjugate base reaction (i.e. SNCB) will take place and there will be retention of configuration and it will be R product. When C5H5NH reacts with reactant SN2 reaction will take place and configuration inversion will take place and it will be S product.

8. What will be the correct order of SN2/E2 ratio for the %yield of the product of the following halide?
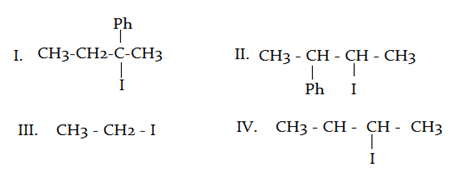
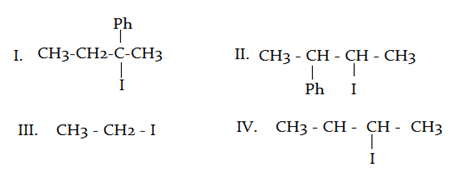
a) III > IV > II > I
b) III > II > IV > I
c) I > III > IV > II
d) II > I > III > IV
View Answer
Explanation: Least hindered halide give fastest SN2 reaction as the hindrance increases. As the hindrance increases, the occurrence of SN2 reaction decreases.
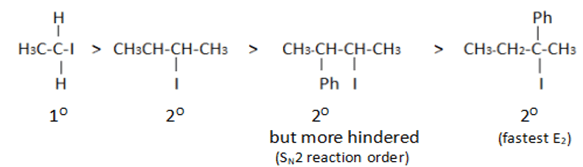
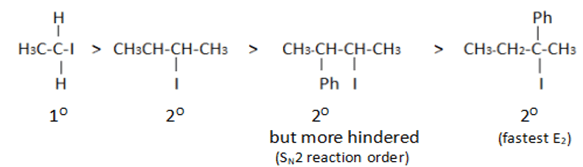
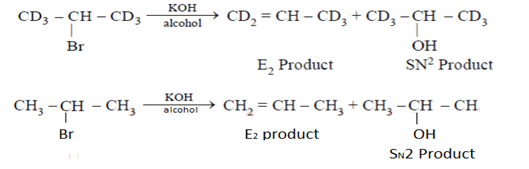
9. Which of the following is an incorrect statement?
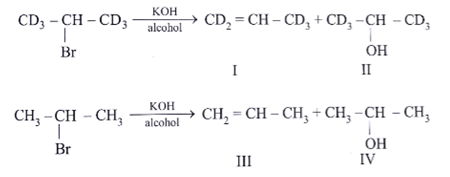

a) Rate of formation of (II) and (IV)would be identical
b) Rate of formation of (I) would be slower than that of (III)
c) Formation of (I) would show primary isotope effect
d) Formation of (III) involves E1 reaction
View Answer
Explanation: Rate of formation of II and IV similar because H & D are approximately of same size.
C–H & C–D bond breaking is the part of RDS hence rate in (I) is slower than (III)
It undergoes E2 not via E1 because carbocation is not stable.

10. Among the following reactions, which one is incorrect?
a) 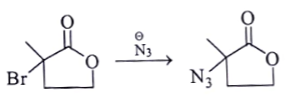

b) 

c) 

d) 

View Answer
Explanation:
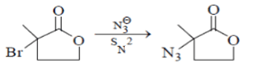
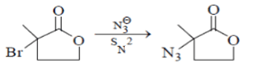
α-halocarbonyl are the best SN2 substrate.
Here nucleophilic substitution reaction will happen and the above product will be formed. Hence this reaction is correct.
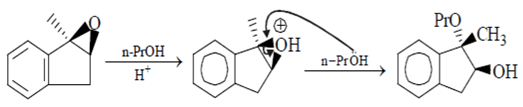

Here (PrO) and (CH3) will be at same carbon atom and (OH) will be at adjacent carbon atom. Hence this reaction is wrong.


n-PrO– will attack the carbon atom attached to oxygen above the plane and the above product will be formed. Hence the reaction is correct.
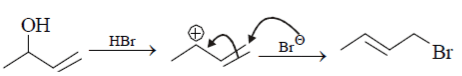
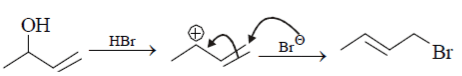
Attack of Bromine ion (Br–) will be at that carbon atom where less substitution is present, the above product will form. Hence this reaction is correct.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
