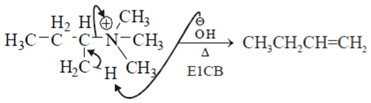
This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Elimination Reaction”.
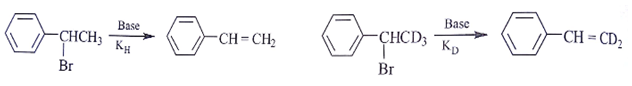
1. Assuming both the reactions as E1, where will the expected ratio between KH/KD lies between?
a) nearly I
b) nearly 3
c) nearly 5
d) anything in between 2 and 8
View Answer
Explanation: In E1 elimination RDS is the formation of carbocation from halide hence C – H and C – D are not the part of RDS there KH/KD is approx. 1.
2. Predict the product for the following elimination reaction.
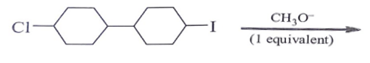
a)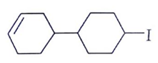
b) 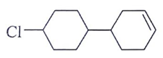
c) 
d) 
View Answer
Explanation: Iodine is a better leaving group than chlorine, so attach will be at iodine site.
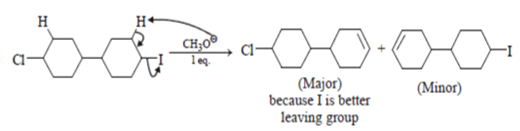
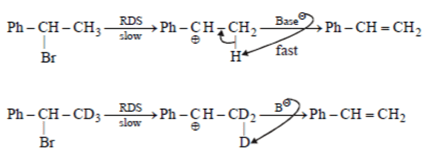
3. Which of the following is incorrect statement for the giver reaction?
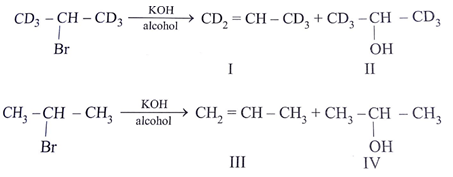
a) Rate of formation of II and IV would be identical
b) Rate of formation of I would be slower than that of III
c) Formation of I would show primary isotope effect
d) Formation of III involves E1 reaction
View Answer
Explanation: Rate of formation of II and IV similar because H & D are approximately of same size.
C–H & C–D bond breaking is the part of RDS hence rate in I is slower than III
It undergo E2 not via E1 because carbocation is not stable.
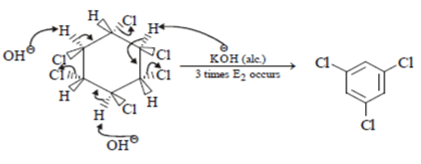
4. When the all-cis isomer of C6H6Cl6 (2, 3, 4, 5, 6-Hexachlorocyclohexane) is heated with alc.KOH, what will be the most probable product?
a) 
b) 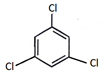
c) 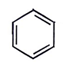
d) ![]()
View Answer
Explanation: Here three times Elimination(E2) occurs and three pie bond will be formed and three water molecule will be removed as shown below.
5. Which of the following statements is correct for alkyl halide?
a) Alkyl halide will always show SN1 mechanism
b) As branching at carbon increases, E1 mechanism is favoured as compared to SN1 mechanism
c) In unimolecular reaction, increasing the temperature donot favours E1 mechanism
d) In most unimolecular reactions of alkyl halide E1 reaction is favoured over SN1 reaction
View Answer
Explanation: In most unimolecular reactions of alkyl halide SN1 reaction is favoured over E1 reaction. E1 mechanism is favoured as compared to SN1 mechanism by branching at carbon increases, as more steric hinderence at the attacking site will leads to E1 mechanism.
6. Which of the following order is incorrect for the rate of E2 reaction?
a) 5-Bromocycloheptene > 4-Bromocycloheptene
b) 2-Bromo- I -phenylbutane > 3-Bromo- I-phenylbutane
c) 3-Bromocyclohexene > Bromocyclohexane
d) 3-Bromo-2-methylpentane > 2-Bromo-4-methylpentane
View Answer
Explanation: For 5-Bromocycloheptene > 4-Bromocycloheptene, more stable product leads to faster rate of reaction. Product formed by E2 of 4-Bromocycloheptene is more stable than 5-Bromocycloheptene. So, rate of reaction 4-Bromocycloheptene is fater than 5-Bromocycloheptene. So option 5-Bromocycloheptene > 4-Bromocycloheptene is incorrect.
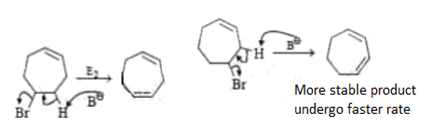
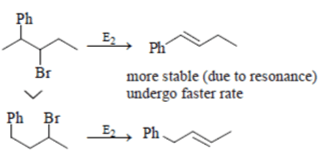
For 2-Bromo- I -phenylbutane > 3-Bromo- I-phenylbutane, it is true rate of reaction for 2-Bromo- I -phenylbutane is more than 3-Bromo- I-phenylbutane as product formed in former is more stable due to resonance.
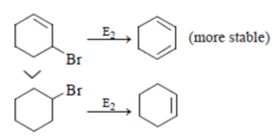
For 3-Bromocyclohexene > Bromocyclohexane, it is true rate of reaction for 3-Bromocyclohexeneis more than Bromocyclohexane as product formed in former is more stable.
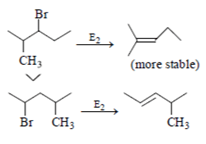
For 3-Bromo-2-methylpentane > 2-Bromo-4-methylpentane, it is true rate of reaction for 3-Bromo-2-methylpentane more stable than 2-Bromo-4-methylpentane as product formed in former is more stable.
7. Which of the following statement is correct?
a) E2 is a concerted reaction in which bonds break and new bonds form at the same time in a single step
b) Order of reactivity of alkyl halides towards E2 dehydrohalogenation is found to be 3o > 2o > 1o
c) In E2 elimination different stereoisomer (diastereomer) converts into different stereo product
d) All of the mentioned
View Answer
Explanation: E2 is a concerted reaction in which bonds break and new bonds form at the same time in a single step. Order of reactivity of alkyl halides towards E2 dehydrohalogenation is found to be 3o > 2o > 1o. In E2 reaction both hydrogen and leaving group should be antiperiplanar and that’s why In E2 elimination different stereoisomer (diastereomer) converts into different stereo product.
8. Which of the following statement is correct regarding the following reaction?
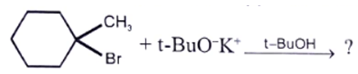
a) Major product is endocyclic alkene formed according to Saytzeff
b) Major product is exocyclic alkene formed according to Saytzeff
c) Major product is exocyclic alkene formed according to Hoffmann
d) Major product is endocyclic alkene formed according to Hoffmann
View Answer
Explanation: Exocyclic alkene are more stable than endo beacuse of but due to “steric hindrance” the less substituted alkene is formed.
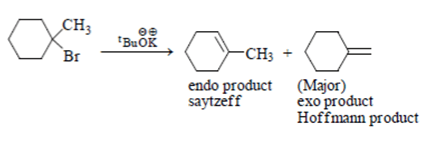
9. Which of the following statement (s) is/are true about the following eliminations?

(1) Hoffmann product is major product in I (2) Saytzeff product is major product in I (3) Hoffmann product is major product in II (4) Saytzeffproduct is major product in II
a) 1 and 2
b) 1 and 4
c) 2 and 3
d) 3 and 4
View Answer
Explanation: Here correct is statement 1 and 4 are true. Due to steric hinderence tert-Butyl alcohol anion will attack less substituted C-H bond.
Ethanol anion will attack more substituted C-H bond to form more stable alkene.
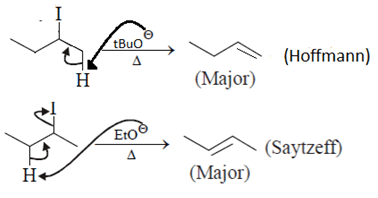
10. Which incorrect about alkyl bromide having molecular formula C5H11Br?
a) One isomeric alkyl bromide undergoes E1 elimination at the fastest rate
b) Only one is incapable of reacting by the E2 mechanism
c) Only one isomer gives a single alkene on E2 elimination
d) 2-Bromopentane gives the most complex mixture of alkenes on E2 elimination
View Answer
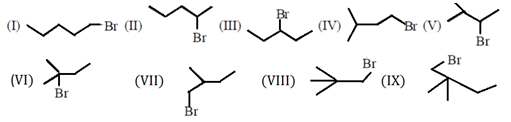
Explanation:
From the following isomers of C5H11Br, except IX all are capable of E2 elimination.
11. In which reaction product formation takes place by hoffmann rule?
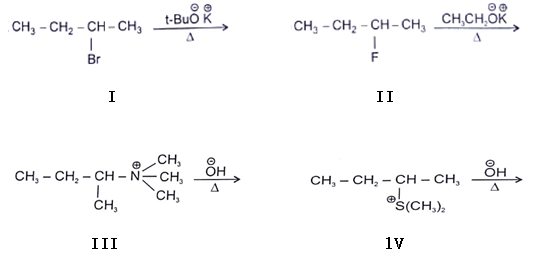
a) I and II
b) II and III
c) I, II and III
d) I, II, III and IV
View Answer
Explanation: All of the above molecules will form product by hoffmann rule that is less substituted C-H bond will be attacked because more stable product will be formed via this in all cases.
I.
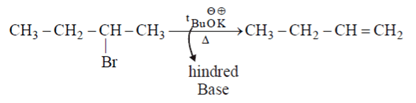
II.
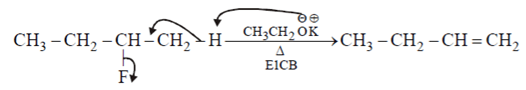
When leaving group are F & NH2 type then they give hoffmann product.
12. Which of the following compounds cannot give E1CB reaction?
a) CF3 – CHCl2
b) C6h5 – CH2 – CH2F
c) CH3 – CH2 – CH2Br
d) 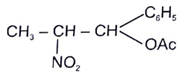
View Answer
Explanation:
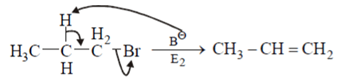
Compound d will give E2 reaction, Br is a better leaving group so formation of carboanion and removal of leaving group occurs simultaneously. But E1CB reaction is shown by poor leaving group like in compounds a, b and c.
13. Which of the following are incorrect statement?
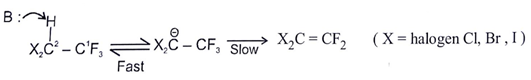
a) X being electronegative, makes the H (on C-2) more acidic
b) Due to electron withdrawal nature of X, it stabilises the carbanion
c) (X) destabilises the carbanion due to the presence of lone pairs
d) The reaction proceeds by an E1CB pathway
View Answer
Explanation: Due to inductive effect of halogen it stabilize the negative charge on adjacent to carbon atom, as X will withdraw the electron towards itself.
14. Which of the following is not the examples of E1CB reaction?
a) 
b)
c) 
d) 
View Answer
Explanation: Compound d will give E2 reaction, CL is a better leaving group so formation of carboanion and removal of leaving group occurs simultaneously. But E1CB reaction shown by poor leaving group like in compounds a, b and c.
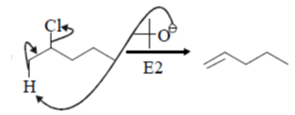
15. Which of the following reaction will not show addition elimination mechanism?
a) 
b) 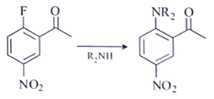
c) 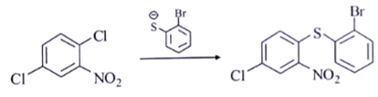
d) 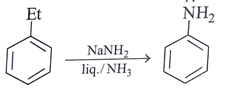
View Answer
Explanation: Compounds a, b and c will follow Addition- Elimination mechanism.
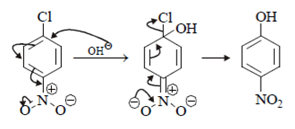
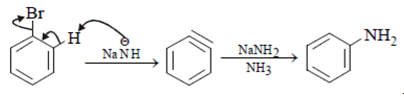
Compound will follow elimination -addition mechanism, as shown in below reaction. NH– anion first attack on the ring which lead to formation of benzyne ring and further addition of -NH2 will take place.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books