This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Rearrangement Reactions”.
1. Which types of isomers are formed in rearrangement reactions?
a) structural isomers
b) Geometrical isomers
c) Optical isomer
d) Conformational isomers
View Answer
Explanation: Products formed have the same molecular formula, but their atoms have different arrangements or bonds. For example, Butane and isobutane have the same number of carbon (C) atoms and hydrogen (H) atoms, so their molecular formulas are the same.
2. What is the main difference between Hofmann and Curtius rearrangement?
a) Products are different
b) Intermediate formed is different
c) Reactants are different
d) Isomers
View Answer
Explanation: The Hofmann rearrangement occurs with an amide. The Curtius rearrangement occurs with an acyl azide.
3. With accompanying 1, 2-rearrangement in wolff rearrangement, an α-diazocarbonyl compound is converted into a ketene by loss of which of the following compound?
a) Dioxygen
b) Dinitrogen
c) Disulphur
d) Ammonia
View Answer
Explanation: The leaving group (N2) and the migrating group (R1) are antiperiplanar, which favors a concerted mechanism, in which nitrogen extrusion occurs concurrently with 1, 2-alkyl shift.
4. Which Intermediate is formed in Wolff’s reaction?
a) Carbene
b) Ketene
c) Carbocation
d) Carbanion
View Answer
Explanation: Ketene is formed as intermediate in Wolff’s reaction. Formation of Diazonium ion will be followed by reaction in presence of heat which leads to rearrangement of bonds and ketene will be formed.

5. Which was the first molecular rearrangement identified as such by early chemists?
a) Wolff’s rearrangement
b) Pinacole rearrangement
c) Favorskii rearrangement
d) Hofmann rearrangement
View Answer
Explanation: The pinacol rearrangement was the first molecular rearrangement identified as such by early chemists.
6. Which intermediate carbocation is more stable in pinacole -pinacolone rearrangement?
a) 1o
b) 2o
c) 3o
d) 4o
View Answer
Explanation: 3o-carbocation is relatively stable, and has been shown to return to pinacol by reaction in the presence of isotopically labeled water. A 1, 2-methyl shift generates an even more stable carbocation in which the charge is delocalized by heteroatom resonance.
7. In which medium Favorskii rearrangement occurs?
a) Acidic
b) Basic
c) Neutral
d) Alkaline
View Answer
Explanation: It is a base catalysed reaction:
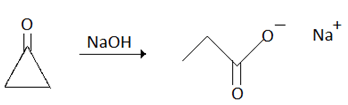
Mechanism of Favorskii rearrangement: Here OH- group of NaOH is attaching at the keto-group and the ring will open for the stability of the molecule.
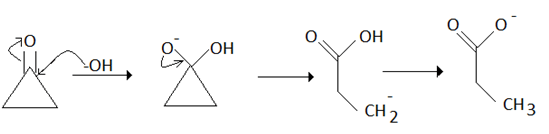
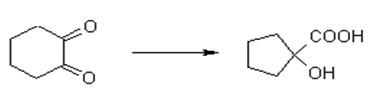
8. The benzylic acid rearrangement reaction of a cyclic diketone leads to _______
a) Ring expansion
b) Ring contraction
c) Ring fusion
d) Isomers
View Answer
Explanation: The benzylic acid rearrangement reaction of a cyclic diketone leads to ring contraction as shown in below diagram.
9. Which medium is used in benzylic acid rearrangement reaction?
a) Neutral
b) Strong basic
c) Mild acidic
d) Strong acidic
View Answer
Explanation: The mechanism of this benzylic acid rearrangement starts with the attack of hydroxide on one of the carbonyl groups.
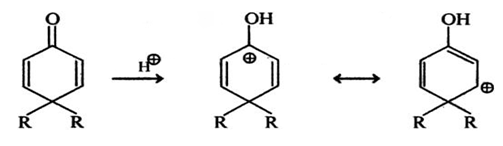
10. Which type of catalytic reaction, does Dienone phenol rearrangement reaction belong?
a) Acid catalysed
b) Base catalysed
c) Acidic
d) Neutral
View Answer
Explanation: The first step in the mechanism of this reaction is the protonation of the most basic atom in the molecule, the oxygen of the carbonyl group (as shown in below reaction).
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
