This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Free Radical Vinyl Polymerisation”.
1. Which of the following is an initiator molecule in the free radical polymerisation?
a) Benzoyl peroxide
b) Sulphuric acid
c) Potassium permanganate
d) Chromium oxide
View Answer
Explanation: The whole process of free radical polymerisation starts off with a molecule called an initiator. This is a molecule like benzoyl peroxide. When they split, the pair of electrons in the bond which is broken will separate. This is unusual as electrons like to be in pairs whenever possible. When this split happens, we’re left with two fragments, called initiator fragments.
2. Why is vinyl polymerization also known as addition polymerization?
a) monomers are unsaturated compounds
b) it is a chain reaction
c) most monomers contain (CH2=CH─) group
d) it proceeds through radical
View Answer
Explanation: In addition polymerisation, polymer are formed by simple linking of monomers without the co-generation of other products. Most of the monomers involves in chain polymerization contains (CH2=CH─) group, known as vinyl group.
3. Which of the following happens in initiation step of the free radical polymerisation?
a) Decomposition of initiator
b) Renewal of inhibitor
c) Addition of monomer molecules to the growing chains
d) Disproportionation
View Answer
Explanation: The breakdown of the initiator molecule to form radicals, followed by the radical’s reaction with a monomer molecule is called the initiation step of the polymerization.
4. Which of the following happens in propagation step of the free radical polymerisation?
a) Decomposition of initiator
b) Renewal of inhibitor
c) Addition of monomer molecules to the growing chains
d) Disproportionation
View Answer
Explanation: The adding of more and more monomer molecules to the growing chains, is called propagation. Self-perpetuating reactions like this one are called chain reactions.
5. Which of the following happens in termination step of the free radical polymerisation?
a) decomposition of initiator
b) addition of free radical to monomer
c) addition of monomer molecules to the growing chains
d) disproportionation
View Answer
Explanation: Termination reaction in which our unpaired electrons can shut down the polymerization: it’s is called disproportionation. This is a rather complicated way in which two growing polymer chains solve the problem of their unpaired electrons.
6. Which of the following reagents may be used to initiate radical polymerization of styrene?
a) HCl
b) Peroxides
c) Hydroxide ion
d) BF3
View Answer
Explanation: The whole process of free radical polymerisation starts off with a molecule called an initiator. This is a molecule like peroxide or AIBN.
7. Which polymer will be formed when vinyl acetate reacts with peroxides?
a) 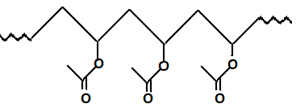
b) 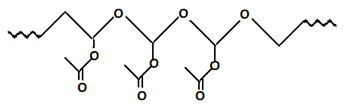
c) 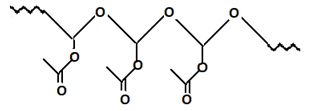
d) 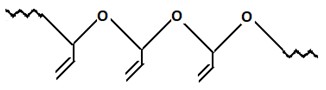
View Answer
Explanation: Polyvinyl acetate, or PVA for short, is one of those low-profile behind-the-scenes polymers. It is found in the case with polyethylene or polystyrene.

8. What is the name for polymers with Z groups only on one side of the carbon chain?
a) Enatiometric
b) Atactic
c) Syndiotactic
d) Isotactic
View Answer
Explanation: Isotactic polymers are composed of isotactic macromolecules. In isotactic Macromolecules all the substituents are located on the same side of the macromolecular backbone.
9. Which of the following is the structure of the radical intermediate formed in the first propagation step of the polymerization of methyl methacrylate?
a) 
b) 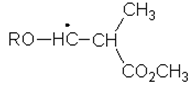
c) 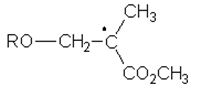
d) 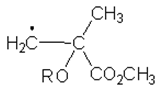
View Answer
Explanation: N, N‐Dimethylaniline (DMA) does initiate the free‐radical polymerization of methyl methacrylate (MMA), resulting into alkyl radicals(shown below) underwent selective addition to the two monomers.

10. How many monomer units of ethylene are present in the given polyethylene formed by?
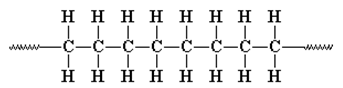
a) 1
b) 2
c) 3
d) 4
View Answer
Explanation: A repeat unit or repeating unit is a part of a polymer whose repetition would produce the complete polymer chain (except for the end-groups) by linking the repeat units together successively along the chain, therefore, here number of monomers are four.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
