This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Acetals”.
1. Which of the following statements is wrong?
a) Hydrolysis of an acetal is catalysed by acids
b) Hydrolysis of an acetal is catalysed by aqueous acid
c) Oximes are stabilized by conjugation between the C=N and OH groups
d) Enamines are formed between secondary amines and the carbonyl group of aldehydes and ketones
View Answer
Explanation: Hydrolysis of an acetal is catalysed by aqueous acid, Acetals are not stable to aqueous acid and are very readily hydrolyzed back to the parent alcohol and carbonyl compound under these reaction conditions.
2. Acetal on acid hydrolysis generates which of the following?
a) Alcohol
b) Amine and aldehyde
c) Ketone and alcohol
d) Ketone and ether
View Answer
Explanation: A series of cyclic and acyclic acetals and ketals were hydrolyzed to their corresponding carbonyl compounds by a catalytic amount of CBr4 (20%) in CH3CN/H2O solvent mixture under different energy sources, thermal or ultrasound.
3. Which combination of an aldehyde and an alcohol most readily forms a hemiacetal with base catalysis?
a) 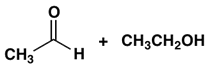
b) 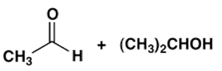
c) 
d) 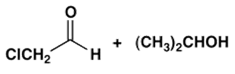
View Answer
Explanation: An electron-withdrawing substituent (e.g., Cl) facilitates nucleophilic addition to the carbonyl of an aldehyde whereas bulky groups in an alcohol lead to steric hindrance in its addition to a carbonyl in the formation of a hemiacetal; for formation of hemiacetals. The balance of these effects in the following leads to being the most reactive system.
4. Which of the following most readily forms a cyclic hemiacetal with acid catalysis?
a) 
b) 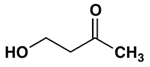
c) 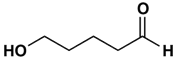
d) 
View Answer
Explanation: Hydroxy aldehydes and ketones of the right carbon chain length form cyclic hemiacetals with acid catalysis; ones leading to a five-membered product usually give the highest yields, and hydroxy aldehydes generally give better yields than hydroxy ketones.
5. Which is normally the main product when a mixture of aldehyde RCHO and an excess of alcohol R’OH is treated with an acid catalyst?
a) 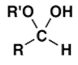
b) 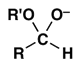
c) 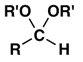
d) 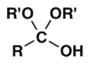
View Answer
Explanation: The reaction conditions given are those for acetal formation.
6. Which is normally the main product when a mixture of aldehyde RCHO and an excess of alcohol R’OH is treated with a base catalyst?
a) 
b) 
c) 
d) 
View Answer
Explanation: In the presence of a base rather than an acid, the reactants go no further than formation of the hemiacetal.
7. Consider the mechanism of the acid-catalysed formation of cyclic acetals from ketones and diols, which of the following structures does not represent a legitimate intermediate in this reaction?
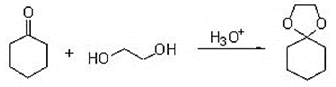
a) 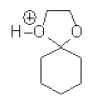
b) 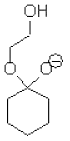
c) 
d) 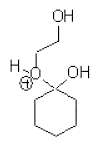
View Answer
Explanation: It does not represent a legitimate intermediate in this reaction, Consider the mechanism of the acid-catalysed formation of cyclic acetals from ketones and diols, it does not form the desired acetal form.
8. Which of the following structures is a hemiacetal?
a)
b) 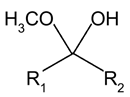
c) 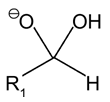
d) 
View Answer
Explanation: An acetal is a functional group with the following connectivity R2C(OR’)2, where both R’ groups are organic fragments.
9. Dimethoxymethane is dimethyl acetal of which of the following?
a) Formaldehyde
b) Acetaldehyde
c) Tolualdehyde
d) Propionaldehyde
View Answer
Explanation: Dimethoxymethane is dimethyl acetal formaldehyde.
10. Which of the following is not polyacetal?
a) Dimethoxymethane
b) Dioxolane
c) Starch
d) Cellulose
View Answer
Explanation: Cellulose is a ubiquitous example of a polyacetal. Dimethoxymethane, dioxolane and starch is an example of a polyacetal.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
