This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Dihydric Alcohols”.
1. How many hydroxyl groups are present in diols?
a) One
b) Two
c) Three
d) Four
View Answer
Explanation: Any of a class of alcohols that have two hydroxyl groups in each molecule are diols or dihydric alcohol.
2. Which of the following is not the example of dihydric alcohol?
a) Glycerin
b) Parahydroxyphenol
c) Resorcinol
d) Catechol
View Answer
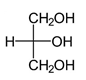
Explanation: Glycerin is a trihydroxy alcohol i.e. Glycerol, is a simple polyol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. Structure of glycerin is
3. Which of the following is the correct structure of catechol?
a) 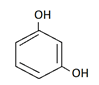
b) 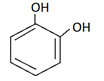
c) 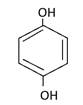
d) 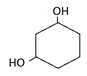
View Answer
Explanation: Catechol is dihydric alcohol, in which hydroxyl group is attached adjacent to each other in benzene ring.
4. Which of the following is the correct structure of parahydroxyphenol?
a) 
b) 
c) 
d) 
View Answer
Explanation: Parahydroxyphenol is dihydric alcohol, in which hydroxyl group is attached at 1, 4 positions to each other in benzene ring.
5. Which of the following is the correct structure of resorcinol?
a) 
b) 
c) 
d) 
View Answer
Explanation: Resorcinol is dihydric alcohol, in which hydroxyl group is attached at 1, 3 positions to each other in benzene ring.
6. What is the general formula for dihydric alcohol?
a) (CH2)n(OH)2 where n = 2,3…etc
b) (CH2)n(OH)2 where n = 1,2,4… etc
c) CnH2n+1OH where n = 1, 2 …etc
d) (CH2)n(OH)3 where n = 3, 4, 5 …etc
View Answer
Explanation: Dihydric alcohol have general formula (CH2)n(OH)2, where n = 2,3,4 etc.
7. Which of the following reagent can be used to carry out this synthesis?
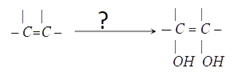
a) Seliwanoff reagent
b) Baeyer’s reagent
c) Barfoed reagent
d) Benedict reagent
View Answer
Explanation: Cold dilute alkaline solution of Bayer’s reagent can be used to carry out this synthesis.
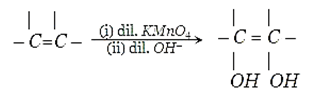
8. Which type of product is formed when Cold dilute alkaline solution of Bayer’s reagent reacts with alkene?
a) Syn-diol
b) Anti-diol
c) syn- and anti-geometry will not be there in diol
d) Trans diol
View Answer
Explanation: On hydroxylation product is formed when Cold dilute alkaline solution of Bayer’s reagent reacts with alkene is syn.
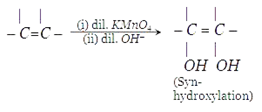
9. What will be the compound A which can be used to carryout this synthesis of diols?
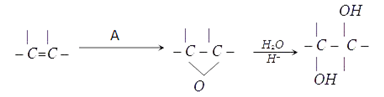
a) RCO2OH
b) RCHO
c) RCOR’
d) RCOOH
View Answer
Explanation: A peroxy acid can be used to carryout this synthesis as shown in below reaction.
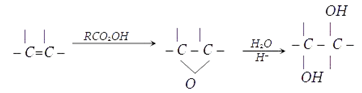
10. How can we detect the presence of resorcinol in the solution?
a) Ceric ammonium nitrate test
b) Lucas test
c) Phthalic acid test
d) Remini’s test
View Answer
Explanation: Phthalic acid test:The anhydrides of aromatic 1,2-dicarboxylic acids on heating with resorcinol gives a dye fluorescein. This dye in NaOH solution gives a yellowish red solution with green fluorescence.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
