This set of Organic Chemistry Assessment Questions and Answers focuses on “Electrophilic Substitution in Pyridine”.
1. Electrophilic substitution reaction of pyridine is most effective in which of the conditions?
a) In slightly acidic conditions
b) In slightly basic conditions
c) In neutral medium
d) In vigorous conditions
View Answer
Explanation: When electrophilic substitution reaction of pyridine is carried out in acidic conditions then pyridine ring will further get deactivated forming pyridinium ion. However, under more vigorous conditions the usual electrophilic substitution reaction can be possible but yield will be less.
2. Which of the following electrophilic substitution reaction is not possible in pyridine?
a) Nitration
b) Sulphonation
c) Bromination
d) Friedel craft reaction
View Answer
Explanation: Common alkylations and acylations, such as Friedel–Crafts alkylation or acylation, usually fail for pyridine because they lead only to the addition at the nitrogen atom. Substitutions usually occur at the 3-position, which is the most electron-rich carbon atom in the ring and is, therefore, more susceptible to an electrophilic addition.
3. At which position of pyridine electrophilic substitution reaction is most preferred?
a) First
b) Second
c) Third
d) Forth
View Answer
Explanation: Electrophilic substitution reaction at position the 3-position is preferred over attack at 2 and 4-position because the intermediate found by electrophilic addition at 3-position is more stable as it will have 3-resonating structure and none of will have positive charge on N.
4. Which of the following is the product for the below reaction?
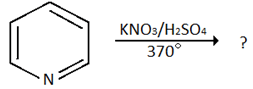
a) 3- Nitropyridine
b) 2- Nitropyridine
c) 4- Nitropyridine
d) 2,4- dinitropyridine
View Answer
Explanation: Electrophilic substitution reaction at position the 3-position is preferred over attack at 2 and 4-position because the intermediate found by electrophilic addition at 3-position is more stable as it will have 3-resonating structure and none of will have positive charge on N.
5. Which of the following is the product for the below reaction?
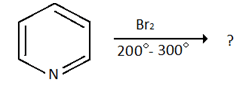
a) 3- bromopyridine
b) 2- bromopyridine
c) 2- bromopyridine and 3- bromopyridine
d) 3- bromopyridine and 3,5- dibromo pyridine
View Answer
Explanation: Electrophilic substitution reaction at position the 3-position 5 position is preferred over attack at 2 and 4-position because the intermediate found by electrophilic addition at 3-position is more stable as it will have 3-resonating structure and none of will have positive charge on N.
6. Which of the following is the product for the below reaction?
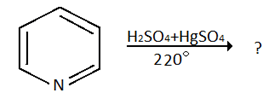
a) 3- pyridinesulphonic acid
b) 2- pyridinesulphonic acid
c) 4- pyridinesulphonic acid
d) 2,4- pyridinedisulphonic acid
View Answer
Explanation: Electrophilic substitution reaction at position the 3-position is preferred over attack at 2 and 4-position because the intermediate found by electrophilic addition at 3-position is more stable as it will have 3-resonating structure and none of will have positive charge on N.
7. Which of the following is the product for the below reaction?
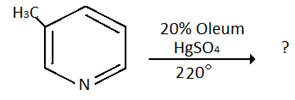
a) 3- methyl-pyridine-5-sulphonic acid
b) 3- methyl-pyridine-4-sulphonic acid
c) 3- methyl-pyridine-6-sulphonic acid
d) 3- methyl-pyridine-1-sulphonic acid
View Answer
Explanation: Alkyl group activate the pyridine ring towards electrophilic substitution, however, directive influence of the ring predominates regardless of the position of alkylation i.e. attack at 3-position.
8. Which of the following is the product for the below reaction?
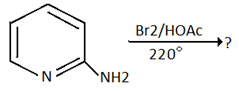
a) 5- bromo-2-aminopyridine
b) 3- bromo-2-aminopyridine
c) 4- bromo-2-aminopyridine
d) 6- bromo-2-aminopyridine
View Answer
Explanation: Amino group dominates the ring orientation effect and direct the incoming electrophile to o- and p- position. 2-amino group directs the incoming electrophile predominantly to the 5-position.
9. Which of the following is the product for the below reaction?
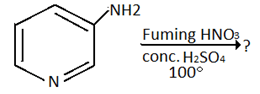
a) 2- Nitro-3-aminopyridine
b) 4- Nitro-3-aminopyridine
c) 5- Nitro-3-aminopyridine
d) 6- Nitro-3-aminopyridine
View Answer
Explanation: Amino group dominates the ring orientation effect and direct the incoming electrophile to o- and p- position. 3-amino group directs the incoming electrophile predominantly to the 2-position.
10. Which of the following is the product for the below reaction?
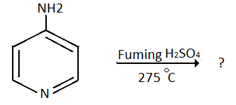
a) 4-amino -pyridine-5-sulphonic acid
b) 4- amino -pyridine-3-sulphonic acid
c) 4- amino -pyridine-6-sulphonic acid
d) 4- amino -pyridine-2-sulphonic acid
View Answer
Explanation: Amino group dominates the ring orientation effect and direct the incoming electrophile to o– and p– position. 4-amino group directs the incoming electrophile predominantly to the 3-position.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry Assessment Questions, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
