This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Aryl Halides”.
1. Dehydrohalogenation of 1,2 di-bromo cyclohexane gives which of the following compound?
a)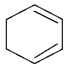
b)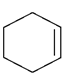
c)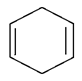
d) 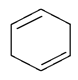
View Answer
Explanation: As we can see in below reaction that double E2 elimination yields the conjugated diene, 1,3-cyclohexadiene. Note the overall transformation is alkene to conjugated diene.
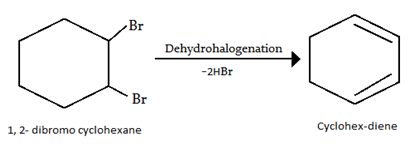
2. Which one of the following possess highest melting point?
a) Chlorobenzene
b) o-dichlorobenzene
c) m-dichlorobenzene
d) p-dichlorobenzene
View Answer
Explanation: p-dichlorobenzene molecule has symmetrical structure. It can fit well in its crystal lattice. The intermolecular forces of attraction are strong. Hence, it possesses highest melting point.
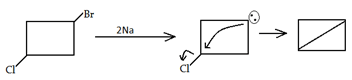
3. What would be the product formed for the following reaction?
a) 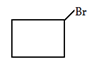
b) 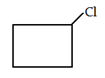
c) 
d) 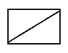
View Answer
Explanation: When 1-Bromo-3-chloro cyclobutane reacts with two equivalents of metallic sodium in ether it is the example of Wurtz reaction.
4. Which of these can be used as moth repellent?
a) Benzene hexachloride
b) Benzal chloride
c) Hexachloroethane
d) Tetrachloroethane
View Answer
Explanation: Hexachloroethane can be used as moth repellent. It has also been used as a plasticizer for cellulose esters in place of camphor, a polymer additive, a component of fungicidal and insecticidal formulations, in the formulation of extreme pressure lubricants, and in the manufacture of fire extinguishing fluids.
5. What will be the product for the following reaction?
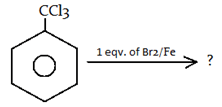
a) 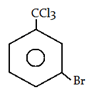
b) 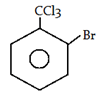
c) 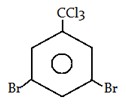
d) 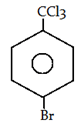
View Answer
Explanation: −CCl3 is a m-directing group. So, attack will be at meta position.
6. Which of the following is true about chlorobenzene?
a) Chlorobenzene is less reactive than benzyl chloride
b) Chlorobenzene is more reactive than ethyl bromide
c) Chlorobenzene is nearly as reactive as methyl chloride
d) Chlorobenzene is more reactive than isopropyl chloride
View Answer
Explanation: Chlorobenzene is less reactive than benzyl chloride. In chlorobenzene the lone pairs present on Cl atom get involved in resonance with π electrons of benzene due to which C−Cl bond acquires double bond character Hence, reactivity decreases. C2H5−Cl is more reactive than C6H5−CH2−Cl.
7. In the below process what is the product A?
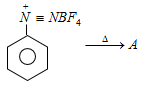
a) Fluorobenzene
b) Benzene
c) 1, 4-difluorobenzene
d) 1, 3-difluorobenzene
View Answer
Explanation: In this reaction aniline is to be treated with nitrous acid to form benzene diazonium chloride, which is further reacted with HBF4 to form benzene diazonium fluorobarate. This is when heated, undergoes decomposition to give fluorobenzene. This reaction is called as Balz-Shiemann reaction.
8. What is the name of the following reaction?
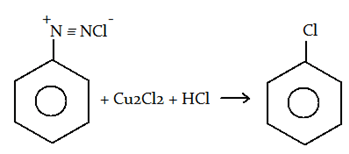
a) Chlorination
b) Sandmeyer’s reaction
c) Perkin reaction
d) Substitution reaction
View Answer
Explanation: The substitution of an aromatic amino group is possible via preparation of its diazonium salt and subsequent displacement with a nucleophile (Cl–, I–, CN–, RS–, HO–) is known as Sandmeyer’s reaction.
9. Which of the following is the commercial method of preparation of Chlorobenzene?
a) Raschig process
b) Wurtz Fitting reaction
c) Friedel-Crafts reaction
d) Grignard reaction
View Answer
Explanation: An industrial process for making chlorobenzene (and phenol) by a gas-phase reaction between benzene vapour, hydrogen chloride, and oxygen (air) at 230°C. The catalyst is copper(II) chloride.
2C6H6 + 2HCl + O2 → 2H2O + 2C6H5Cl
10. Benzene reacts with chlorine to form benzene hexachloride in presence of which of the following reactant?
a) Nickel
b) AlCl3
c) Bright sunlight
d) Zinc
View Answer
Explanation: Benzene will react with three molecules of chlorine in the presence of sunlight to give benzene hexachloride.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
