This set of Organic Chemistry Quiz focuses on “Infrared Spectroscopy – 2”.
1. Why in the IR spectrum of Benzoyl chloride, a weak band near 1750 cm-1 is formed?
a) Inductive effect
b) Fermi resonance between C = O band and first overtone
c) Conjugation effect
d) Hyperconjugation effect
View Answer
Explanation: Benzoyl chloride
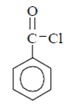
A weak band near 1750 cm-1. Since, band is of weak intensity, it must be due to fermi Resonance. So correct option is Fermi resonance between C = O band and first overtone.
2. Which compound having molecular formula C5H10 shows absorption at 1380 cm-1?
a) 2-Methyl-1-butene
b) Cyclopentane
c) Pentyne
d) Methylcyclobutane
View Answer
Explanation: C5H10→1380 cm-1. 1380 cm-1 represents a C–C bond band. All have C – C bond. Option Pentyne does not fulfill the H-atom requirement. So, correct option is Cyclopentane.
3. Why ketenes absorb in IR at a very high frequency (2150 cm-1)?
a) The inner C is sp hybridized
b) The more s character in a bond, the stronger it is
c) Inner C is sp2 hybridized
d) Inner C is sp3 hybridized
View Answer
Explanation: Ketenes absorb in IR at a very high frequency (2150 cm-1) because inner C is sp2 hybridized. Bonds with more s character absorb at a higher frequency.
4. What is the effect of ring strain in lactone (cyclic ester) or a lactam (cyclic amide)?
a) Increases carbonyl stretching frequency
b) Decreases carbonyl stretching frequency
c) Increases C = C frequency
d) Decreases C = C frequency
View Answer
Explanation: Carbonyl stretching order in both the compounds is 6 > 5 > 4 > 3. Ring strain increases, carbonyl frequency increases.
5. A compound C8 H6 decolorizes Br2 in CCl4 and gives a white precipitate with Tollen’s reagent. It has sharp band at 3300 cm-l and weak bands at 3085, 2110 cm-l. What is this compound?
a) Phenyl acetylene
b) Phenyl propylene
c) Phenyl ethylene
d) Octene
View Answer
Explanation: C8 H6 → Sharp band at 3300 cm-l
Weak band at 3085, 2110 cm-l
Double bond equivalent = C + 1 – H⁄2 – X⁄2 + N⁄2,
(where C→ Carbon, H→ Hydrogen, X→ Halogen, N→ Nitrogen)
= 8 + 1 – 6⁄2 = 6.
One double bond equivalent represents either one bond or one ring.
Now, band at 3300 shows C–H bond, 2110 may be because of C≡C triple bond
Now, we check each option one by one
Option Octene → octene → does not match m.f. C8 H6.
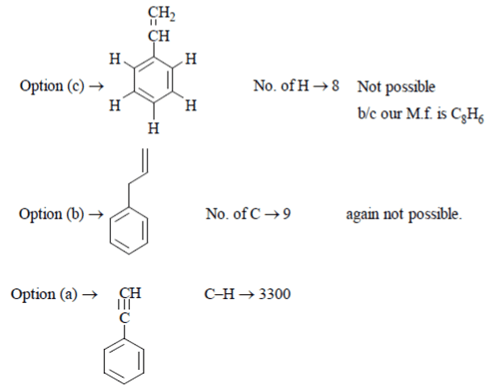
C ≡ C → 2110
D.B.E. → 5πbond
M.f. → C8 H6. Hence, the correct option is Phenyl acetylene.
6. What is the relation between wave number of IR absorption and the reduced mass?
a) Wave number is directly proportional to reduced mass
b) Wave number is inversely proportional to reduced mass
c) Wave number is independent of the reduced mass
d) Wave number is directly proportional to square of reduced mass
View Answer
Explanation: v ∝ 1⁄√μ, frequency is directly proportional to wave number. So, wave number is inversely proportional to reduced mass as shown in the above relation.
7. What is the number of vibrational degrees of freedom in C6H5CH3?
a) 39
b) 15
c) 18
d) 40
View Answer
Explanation: C6H5CH3 is nonlinear.
No. of vibration degree of freedom = 3N – 6
= 3(15) – 6 = 39.
8. Which of the following molecules will not show infrared spectrum?
a) H2
b) HCI
c) CH4
d) H20
View Answer
Explanation: Correct option is H2 as HH2 do not have dynamic dipole moment, so no spectrum will be observed.
9. The phosphorescence spectrum of the excited species is due to which transition?
a) Singlet to triplet transitions
b) Triplet to singlet transitions
c) Vibration modes
d) Electron spin transitions
View Answer
Explanation: The phosphorescence spectrum of the excited species is due to triplet to singlet transitions. In an excited singlet state, the electron is promoted in the same spin orientation as it was in the ground state (paired). In a triplet excited stated, the electron that is promoted has the same spin orientation (parallel) to the other unpaired electron.
10. Why Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1?
a) Mesomeric (M) effect is dominant in acids over the inductive (I) effect
b) I effect is dominant in carboxylic acids over the mesomeric effect
c) I effect on ketones is dominant over the M effect
d) M effect in ketones is dominant
View Answer
Explanation: Monomeric saturated aliphatic carboxylic acids show carbonyl stretching frequency near 1760 cm-1, while saturated aliphatic ketones near 1720 cm-1 because I effect is dominant in carboxylic acids over the mesomeric effect.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry for Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Organic Chemistry Books
