This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Nucleophiles”.
1. Which of the following is not true about nucleophile?
a) donates an electron pair to an electrophile to form a chemical bond
b) all molecules or ions with a free pair of electrons or at least one pi bond can act as nucleophiles
c) nucleophile are Lewis acids by definition
d) a nucleophile becomes attracted to a full or partial positive charge
View Answer
Explanation: Because nucleophiles donate electrons, they are by definition Lewis bases.
2. Which halogen nucleophile is weakest in polar, aprotic solvents?
a) I–
b) F–
c) Cl–
d) Br–
View Answer
Explanation: In polar, aprotic solvents, I– the weakest and this is reversed in polar, protic solvents.
3. Which of the following nucleophile can be used for partial enolization 1,3-Dicarbonyl compound?
a) Lithium di-isopropyl amide (LDA)
b) Et-O–
c) Weak base like NaOH
d) Sodium acetate
View Answer
Explanation: Sodium acetate will do partial enolization of 1,3-Dicarbonyl compound. Weak base like NaOH and Et-O– will form an alcohol and LDA will do complete enolization.
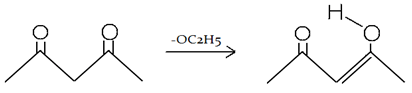
4. What will be the number of products (excluding stereoisomers) in the given reaction?
C6H5CHO + CH3-CHO + NaOH → Product
a) One
b) Three
c) Two
d) Four
View Answer
Explanation: First product will be formed by cross aldol where nucleophile (–OH) will attack at different type or reactant molecule.
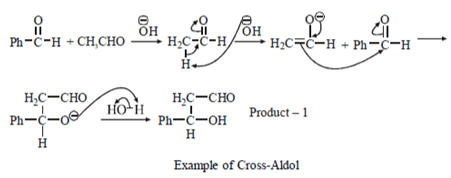
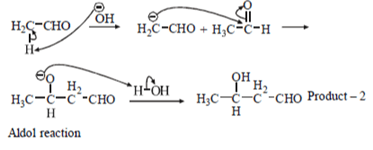
Second type of product will be formed aldol condensation where reaction will occur between same type of molecule.
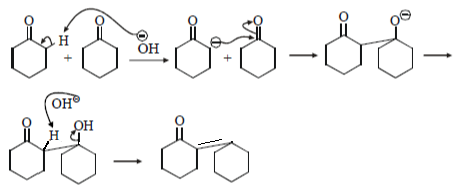
5. Where will nucleophile (-OH) will attack to form which of the following the product X?
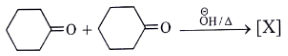
a) 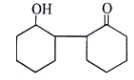
b) 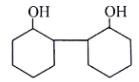
c) 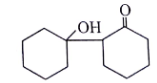
d) 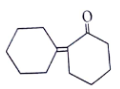
View Answer
Explanation: Nucleophile (-OH) will attack at carbon adjacent to carbonyl bond and form the below product.
6. Which reagent is a good nucleophile?
a) NH3
b) BH3
c) Br2
d) HBr
View Answer
Explanation: Less electronegative atoms make better nucleophiles as they are more willing to share electrons.
7. Which of the following statements is true about ammonia and water?
a) ammonia is more basic and more nucleophilic than water
b) ammonia is less basic and less nucleophilic than water
c) ammonia is more basic but less nucleophilic than water
d) ammonia is less basic but more nucleophilic than water
View Answer
Explanation: Because oxygen is more electronegative than nitrogen, it holds onto its lone pairs more tightly than nitrogen, and hence is less likely to donate its lone pairs to form a covalent bond with a carbon atom during a nucleophilic attack.
8. Which of the following statements is true about the following two anionic molecules?

a) I is more basic and more nucleophilic than II
b) I is less basic and less nucleophilic than II
c) I is more basic but less nucleophilic than II
d) I is less basic but more nucleophilic than II
View Answer
Explanation: Larger atoms make better nucleophiles due to polarizability so II containing S will be more nucleophilic than I but less basic.
9. Which halide ion is the best nucleophile in dimethyl sulfoxide solution?
a) I–
b) F–
c) Cl–
d) Br–
View Answer
Explanation: In polar, aprotic solvents, F– strongest is the nucleophile, and I– the weakest.
10. Which of the following cannot react as a nucleophile?
a) CH3NH2
b) (CH3)2NH
c) (CH3)3N
d) (CH3)4N+
View Answer
Explanation: Nucleophile donates electron to the electrophiles and there is no lone pair present on nitrogen (CH3)4N+, so donation of electron is not possible.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
