This set of Organic Chemistry online quiz focuses on “Chemical Properties of Amines – 2”.
1. A primary amine can be converted to an alcohol by the action of which of the following?
a) Alkali
b) Nitrous acid
c) Reducing agent
d) Oxidizing agent
View Answer
Explanation: A primary amine can be converted to an alcohol by the action nitrous acid.
R – NH2 + HNO2 → R – OH + N2 + H2O
2. The amine which can react with C6H5−SO2−Cl to form a product insoluble in alkali shall be, is which of the following?
a) Primary amine
b) Secondary amine
c) Tertiary amine
d) Both primary and secondary amines
View Answer
Explanation: C6H5SO2Cl is called Hinsberg’s reagent they react with sec amine to form a product in soluble in alkalies. This reaction used to separate primary, secondary and tertiary amine from their mixture.
3. A mixture of benzene and aniline can be separated by which of the following?
a) Hot water
b) dil. HCl
c) dil. NaOH
d) Alcohol
View Answer
Explanation: A mixture of benzene and aniline can be separated by dil. HCl. The mixture is treated with dil. HCL. Only aniline dissolves. It is then shaken with ether. Nitrobenzene goes into ether layer. It is separated.
4. An organic amino compound reacts with aqueous nitrous acid at low temperature to produce an oily nitroso amine. What is this compound?
a) CH3NH2
b) CH3CH2NH2
c) CH3CH2NH.CH2CH3
d) (CH3CH2)3
View Answer
Explanation: Secondary amines gives oily nitrosamine with nitrous acid.
(CH3CH2)2NH + HONO → (CH3CH2)2N.NO + H2O
5. Reaction of aniline with benzaldehyde is which type of reaction?
a) Polymerisation
b) Condensation
c) Addition
d) Substitution
View Answer
Explanation: Reaction of aniline with benzaldehyde is a condensation reaction. Aniline react by initially attacking the carbonyl carbon, followed by hydrogen transfers to give an amine where the nitrogen is bonded to a carbon that also contains a hydroxy group. Under acidic conditions, the hydroxyl group is protonated, followed by loss of water to give the conjugate acid of the observed product(imine). The condensation step to give water is acid catalyzed and is the rate-determining step of the sequence.
6. In the reaction, what is the compound C6H5N=CHC6H5 is known as?
C6H5CHO + C6H5NH2 → C6H5N=HCC6H5 + H2O
a) Aldol
b) Schiffs reagent
c) Schiffs base
d) Benedict reagent
View Answer
Explanation: Aniline react by initially attacking the carbonyl carbon (benzaldehyde), followed by hydrogen transfers to give an amine where the nitrogen is bonded to a carbon that also contains a hydroxy group. Under acidic conditions, the hydroxyl group is protonated, followed by loss of water to give the conjugate acid of the observed product(imine). Hydrogen ion transfer then gives the Schiff base product. The condensation step to give water is acid catalyzed and is the rate-determining step of the sequence.
7. Electrophilic substitution of aniline with bromine in presence of gacial acetic acid gives which of the following?
a) 1, 4, 6-tribromo aniline
b) 2, 4, 6-tribromo aniline
c) 4-bromo aniline
d) 3-bromo aniline
View Answer
Explanation: In water,bromine is ionised up to greater extent to form large number of bromonium ions.Hence Br+ ions attack on aniline from either side to form 2,4,6,-tribromoaniline. In order to obtain monobromo aniline, reaction is carried out in presence of acetic acid. In glacial acetic acid, bromine is ionised to lesser extent and hence relatively less number of bromonium ions are formed.Thus interference of water is ceased.
8. During acetylation of amines what is replaced by acetyl groups?
a) Hydrogen atom attached to nitrogen atom
b) One or more hydrogen atoms attached to carbon atom
c) One or more hydrogen atoms attached to nitrogen atom
d) Hydrogen atoms attached to either carbon atom or nitrogen atom
View Answer
Explanation: During acetylation of amines One or more hydrogen atoms attached to nitrogen atom are removed.
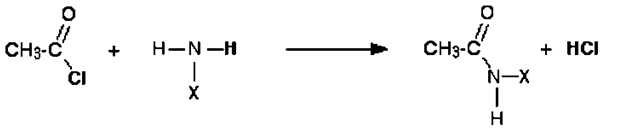
9. Ethyl amine on acetylation gives which of the following?
a) N-ethyl acetamide
b) Acetamide
c) Methyl acetamide
d) Propyl acetamide
View Answer
Explanation: Ethyl amine on acetylation gives N-ethyl acetamide.
CH3CH2NH2 + CH3COCl → CH3CH2NHCOCH3 + HCl
10. p-chloro aniline and anilinium hydrogen chloride can be distinguished by which of the following?
a) Sandmaeyer reaction
b) Carbyl amine reaction
c) Hinsberg’s reaction
d) AgNO3
View Answer
Explanation: Anilinium hydrogen chloride produces chloride ion which gives white precipitate with AgNO3. In fact, anilium chloride is a part of aniline.
11. Identify the product Z in the following reaction.
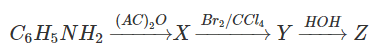
a) p-Bromoaniline
b) p -Bromoacetophenone
c) o-Bromoacetophenone
d) o-Bromoacetanilide
View Answer
Explanation: p-Bromoaniline is formed. For the protection of p- position for bromination, reaction of aniline with acetic anhydride is done and followed by bromination of the product (acetanilide). And then removal of protective group in p-bromoacetanilide is done by hydrolysis, and p- Bromoaniline is formed.
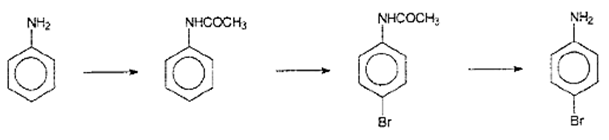
12. Aniline reacts with which of these to form Schiff base?
a) Acetic acid
b) Benzaldehyde
c) Acetone
d) NH3
View Answer
Explanation: Aniline reacts with benzaldehyde and forms Schiff’s base (benzal aniline) or anils.
13. Reaction of cyclohexanone with dimethylamine in the presence of catalytic amount of an acid forms a compound if water during the reaction is continuously removed. What is the compound formed is generally known as?
a) A Schiff’s base
b) An enamine
c) An imine
d) An amine
View Answer
Explanation: Reaction of cyclohexanone with dimethylamine in the presence of catalytic amount of an acid forms a compound if water during the reaction is continuously removed. This compound is known as enamine.
14. Nitration of aniline also gives m-nitro aniline, in strong acidic medium because of which of the following reasons?
a) In electrophilic substitution reaction amino group is meta directive
b) Inspite of substituents nitro group always goes to m- position
c) In strong acidic medium, nitration of aniline is a nucleophic substitution reaction
d) In strong acidic medium aniline present as anilinium ion
View Answer
Explanation: The reason for this is that, in acidic condition protonation of −NH2 group gives anilinium ion (+NH3), which is of deactivating nature and of m-directive nature.
15. The reductive amination of an aldehyde (e.g. the reaction of propanal with ethylamine) can be carried out in the laboratory using which of the following as a reducing agent?
a) NaBH3CN
b) LiAlH4
c) BH3
d) NaBH4
View Answer
Explanation: The reductive amination of an aldehyde (e.g. the reaction of propanal with ethylamine) can be carried out in the laboratory by using NaBH3CN reagent.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry for online Quizzes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
