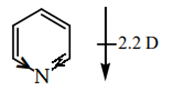This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Structure of Pyridine”.
1. Pyridine is a which type of heterocyclic compound from the following options?
a) Six membered heterocyclic compound
b) Seven membered heterocyclic compound
c) Four membered heterocyclic compound
d) Five membered heterocyclic compound
View Answer
Explanation: Pyridine is a five membered heterocyclic compound. The chemical formula for pyridine is: C5H5N. The only way we can have five carbons, a nitrogen, and only five hydrogens is if the carbons and nitrogen form a ring with alternating double bonds.
2. Which element is present as hetero atom in pyridine?
a) Sulphur
b) Nitrogen
c) Oxygen
d) Sulphur and nitrogen
View Answer
Explanation: Pyridine is an analogue of benzene in which one -CH unit is replaced by N. The chemical formula for pyridine is: C5H5N.
3. The electron of Nitrogen participating in the resonance in pyridine is present in which orbital?
a) p-orbital
b) sp2-orbital
c) sp3-orbital
d) sp -orbital
View Answer
Explanation: In pyridine, from three sp2 orbital, one hybrid orbital is used for one electron which is utilized in the π-cloud.
4. Pyridine is a not a planner compound?
a) True
b) False
View Answer
Explanation: Pyridine is planner monocyclic compound and contains 6 πe- the aromatic sextet. It fulfils the criteria for the Huckel’s rule.
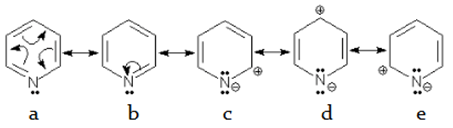
5. How many number of resonating structure stabilises a pyridine molecule?
a) 4
b) 5
c) 6
d) 7
View Answer
Explanation: Like benzene, pyridine also has 2 kekule structure (a and b) along with 3 more resonating structure (c, d and e) which contributes to a lesser extent to the stability of molecule.
6. Pyridine come under which category of heterocycle classification on the basis of chemical behavior?
a) -excessive heterocycle
b) -deficient heterocycle
c) -equivalent heterocycle
d) can’t say about the five membered rings
View Answer
Explanation: Nitrogen is more electronegative than other carbon atom in pyridine, so distribution of 6 e– is unequal (more electron on N).
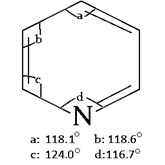
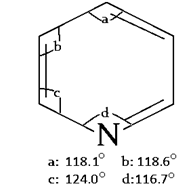
7. What is the smallest angle in pyridine ring?
a) 116.7°
b) 115.0°
c) 124.0°
d) 118.1°
View Answer
Explanation: As shown in below diagram, the angle between C-N-C is the smallest angle which is 116.7°.
8. What is the dipole moment of the pyridine?
a) Zero
b) 2.2 D
c) 1.17 D
d) 4.3 D
View Answer
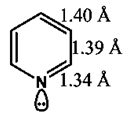
9. Which of the following is the correct range for the bond length in the pyridine molecule?
a) 1.34 – 1.40 A°
b) 1.24 – 1.32 A°
c) 2.4 – 2.49 A°
d) 1.02 – 1.17 A°
View Answer
Explanation: As shown in below diagram, 1.34 – 1.40 A° is the correct range for the bond length in the pyridine molecule.
10. What is the greatest angle in pyridine ring?
a) 116°
b) 140°
c) 124°
d) 118°
View Answer
Explanation: As shown in below diagram, the angle between C-C-N is the greatest angle which is 124°.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship