This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Electrophiles”.
1. Which of the following statement is incorrect about electrophiles?
a) Electrophiles are positively charged or neutral species having vacant orbitals
b) The electrophiles are attacked by the most electron-populated part of one nucleophile
c) Chemical species that do not satisfy the octet rule such as carbenes and radicals are electrophiles
d) Electrophiles are Lewis base
View Answer
Explanation: Because electrophiles accept electrons, they are Lewis acids not Lewis base, according to Acid-Base reaction theories.
2. In addition of halogen (Bromine) to an alkene, how can we isolate a bromonium in the reaction?
a) increasing concentration of bromide ion
b) decreasing concentration of bromide ion
c) alkene with cation stabilising groups
d) alkene with less electrophilic centre
View Answer
Explanation: A bromo-carbenium ion intermediate may be predominant instead of vicinyl dibromide if the alkene has a cation-stabilizing substituent like phenyl group. There is an example of the isolation of the bromonium ion.
3. Which of the following is not an electrophile?
a) (CH3)4N+
b) Cl2
c) HBr
d) Br2
View Answer
Explanation: The ammonium ion cannot react at the N with a nucleophile because the N already has an octet of electrons.
4. Which of the following is not an electrophile?
a) H2O
b) Cl2
c) HBr
d) Br2
View Answer
Explanation: Hydrogen bromide is a strong acid, it is able to donate a proton to a molecule or anion with a nucleophilic site.
5. In the given molecule where will electrophile will attack?
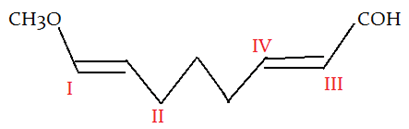
a) I
b) II
c) III
d) IV
View Answer
Explanation: -CH3O is a +M group, so it has lone pair to donate to upcoming electrophile.
6. In the given molecule where will electrophile will attack?
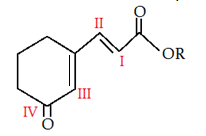
a) I
b) II
c) III
d) IV
View Answer
Explanation: -OCOR is a +M group, it is more electron rich than rest of the groups, so it has lone pair to donate to upcoming electrophile.
7. Which of the following is most readily undergo electrophilic attack?
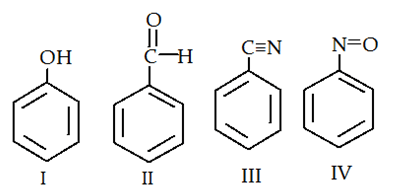
a) I
b) II
c) III
d) IV
View Answer
Explanation: I is most reactive towards electrophilic attack, as -OH is +M group therefore, here benzene will have more e- density than rest of the others.
8. What is the correct order for the rate of reaction for the electrophilic attack of the given compounds?
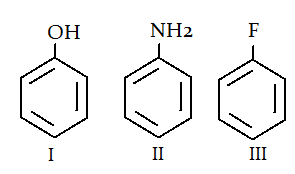
a) I > II > III
b) II > I > III
c) I < II < III
d) III > I > II
View Answer
Explanation: Electronegativity trend is N < O < F, so III is the least electron rich ring therefore least favourable for electrophilic attack. Similarly, II is the most electron rich ring therefore most favourable for electrophilic attack.
9. Which of the following is the product for the given reaction?
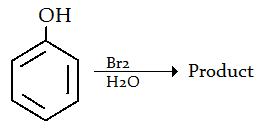
a) 3-Bromophenol
b) 4- Bromophenol
c) 3,5-Dibromophenol
d) 2,4,6-Tribromophenol
View Answer
Explanation: The polar solvent (i.e. water) induces a stronger dipole in Br-Br. A stronger electrophile (Br+) is produced that undergoes electrophilic aromatic substitution with the benzene ring more readily. Consequently, you get tri-bromination with Br(aq.).
10. Which ring will readily undergo electrophilic attack?
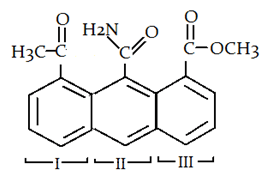
a) I
b) II
c) III
d) Both I and III
View Answer
Explanation: Due to the presence of -NH2 group electron will be transferred to the C of -CONH2 group and that’s why it will not withdraw electron from benzene ring. So, electron density of ring II is most in all three rings and that is why electrophile will attack at ring II.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
