This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Aldehydes”.
1. Primary alcohol is gently heated to produce aldehyde in presence of acidified solution of which of the following compound?
a) hydroxide
b) dichromate
c) ethanol
d) ethanal
View Answer
Explanation: The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromate(VI) acidified with dilute sulfuric acid. If oxidation occurs, then the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions.
2. In the given reaction, what will be the product P?
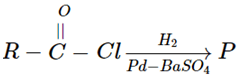
a) RCH2OH
b) RCOOH
c) RCHO
d) RCH3
View Answer
Explanation: The catalytic hydrogenation of acid chlorides allows the formation of aldehydes, is known as Rosenmund reaction.
3. What is the name of the given reaction of preparation of aldehyde?
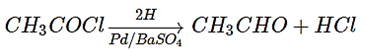
a) Reimer-Tiemann reaction
b) Cannizzaro reaction
c) Rosenmund reaction
d) Reformatsky reaction
View Answer
Explanation: The catalytic hydrogenation of acid chlorides allows the formation of aldehydes, is known as Rosenmund reaction.
4. Which Catalyst is used in Rosenmund reduction?
a) Pd / BaSO4
b) Zn-Hg couple
c) LiAlH4
d) Ni/H2
View Answer
Explanation: The catalytic hydrogenation of acid chlorides allows the formation of aldehydes, is known as Rosenmund reaction. The Pd catalyst must be poisoned, for example with BaSO4, because the untreated catalyst is too reactive and will give some overreduction. Some of the side products can be avoided if the reaction is conducted in strictly anhydrous solvents.
5. On heating calcium acetate and calcium formate, the product formed is which of the following?
a) CH3COCH3
b) CH3CHO
c) HCHO+CaCO3
d) CH3CHO+CaCO3
View Answer
Explanation: Calcium acetate and calcium formate decomposes on heating to form aldehyde and calcium carbonate.
6. In the Rosenmund’s reduction, BaSO4 taken with catalyst Pd acts as which of the following?
a) Promotor
b) Catalytic poison
c) Cooperator
d) Absorber
View Answer
Explanation: The Pd catalyst must be poisoned, for example with BaSO4, because the untreated catalyst is too reactive and will give some overreduction. Some of the side products can be avoided if the reaction is conducted in strictly anhydrous solvents.
7. Catalyst SnCl2/HCl is used in which of the following method of synthesis of aldehyde?
a) Stephen’s reduction
b) Cannizzaro reaction
c) Clemmensen’s reduction
d) Rosenmund’s reduction
View Answer
Explanation: Stephen aldehyde synthesis reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O).
8. What is the product X in the following reaction?
![]()
a) C6H5CH3
b) C6H5CH2Cl
c) C6H5CHO
d) C6H5COOH
View Answer
Explanation: Aldehyde will be formed when benzene is reacted with HCl and carbon monoxide in presence of anhydrous aluminium chloride.
9. Which of the following gases when passed through warm dilute solution of H2SO4 in presence of HgSO4 gives acetaldehyde?
a) CH4
b) C2H6
c) C2H4
d) C2H2
View Answer
Explanation: C2H2 gas when passed through warm dilute solution of H2SO4 in presence of HgSO4 gives acetaldehyde.
10. O3 reacts with CH2=CH2 to form ozonide. On hydrolysis it forms which of the following?
a) Ethylene oxide
b) HCHO
c) Ethylene glycol
d) Ethyl alcohol
View Answer
Explanation: O3 reacts with CH2=CH2 to form ozonide. On hydrolysis it forms HCHO. Ozone gas is passed into a solution of the alkene in some inert solvent like carbon tetrachloride; evaporation of the solvent leaves the ozonide as a viscous oil. This unstable, explosive compound is not purified, but is treated directly with water, generally in the presence of a reducing agent. If oxidsing reagent is used, aldehyde if oxidisable can further oxidise into carboxylic acid which is not the case with reducing agents.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
