This set of Basic Organic Chemistry Questions and Answers focuses on “Nucleophilic Addition Reactions”.
1. What type of reaction takes place upon treatment of a ketone with HCN to form a cyanohydrin?
a) Nucleophilic addition
b) Nucleophilic substitution
c) Electrophilic addition
d) Electrophilic substitution
View Answer
Explanation: The atoms of HCN add to the carbon-oxygen double bond of the ketone by nucleophilic attack of the cyanide anion on the electrophilic carbon of the carbonyl.
2. What is the major organic product obtained from the following reaction?
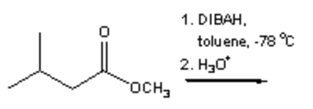
a) 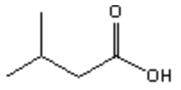
b) 
c) 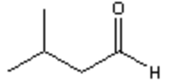
d) 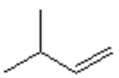
View Answer
Explanation: Di-isobutyl aluminium hydride (DIBAH) is a selective reducing agent. It does not reduce esters to 1° alcohols (lithium aluminium hydride can be used to reduce esters to 1° alcohols).
3. What is the major organic product obtained from the following reaction?

a) 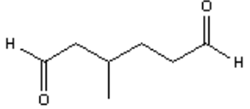
b) 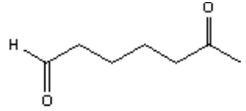
c) 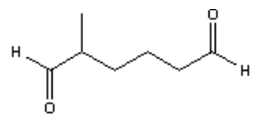
d) 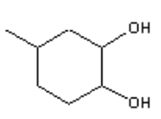
View Answer
Explanation: Ozonolysis of an alkene results in cleavage of the carbon-carbon double bond to form two carbonyl bonds. Ozonolysis of a cyclic alkene in which the double bond has a hydrogen atom on each of the carbon atoms of the carbon-carbon double bond will lead to the formation of a dialdehyde.
4. What is the major organic product obtained from the following reaction?
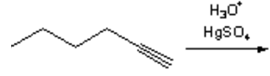
a)
b) 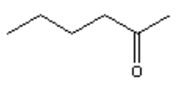
c) 
d) 
View Answer
Explanation: Treatment of an alkyne with aqueous acid in the presence of Hg2+ results in a hydration reaction to form an enol, which tautomerizes to form a ketone. The regiochemistry of the addition is that predicted by Markovnikov’s rule, with the oxygen adding to the more substituted end of the carbon-carbon triple bond.
5. What is the major organic product obtained from the following reaction?
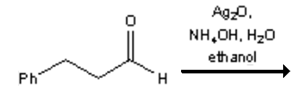
a) 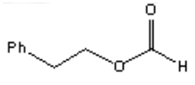
b)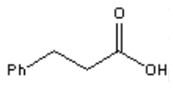
c) 
d) ![]()
View Answer
Explanation: Silver(I) oxide in aqueous ammonia (“Tollens reagent”) is a mild oxidizing agent which oxidizes aldehydes to carboxylic acids without reacting with carbon-carbon double bonds or many other functional groups.
6. What is the major organic product obtained from the following reaction?
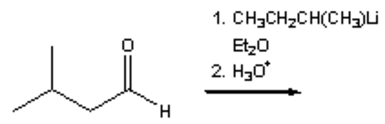
a) 2,4-dimethyl-4-heptanol
b) 4,7-dimethyl-4-heptanol
c) 3,5-dimethyl-4-heptanol
d) 3,5-dimethyl-3-heptanol
View Answer
Explanation: It is also important that products are identified accurately using IUPAC nomenclature: 4,7-Dimethyl-4-heptanol is not an IUPAC name.
7. What is the major organic product obtained from the following reaction?

a) 4-hydroxy-2-pentanone
b) 2-pentanol
c) 2-pentanone
d) 4-penten-2-ol
View Answer
Explanation: Lithium aluminium hydride (LiAlH4) is a reducing agent which reacts with ketones to give 2° alcohols. It does not react with carbon-carbon double bonds.
8. What is the major organic product obtained from the following reaction?
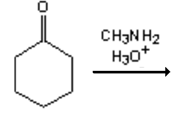
a) 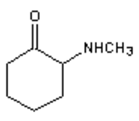
b) 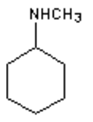
c) 
d) 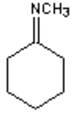
View Answer
Explanation: 1° Amines react with ketones by nucleophilic addition to form a carbinolamine intermediate which undergoes dehydration to give an imine, not an 2° amine.
9. What is the major organic product obtained from the following reaction?
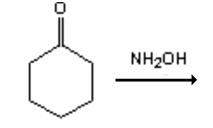
a) 
b) 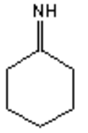
c) 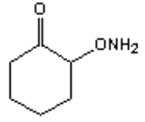
d) 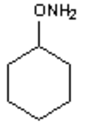
View Answer
Explanation: Hydroxylamine reacts with ketones by nucleophilic addition followed by elimination to give an oxime.
10. What is the major organic product obtained from the following reaction?
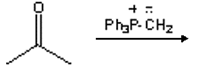
a) 2-methylpropene
b) 2-butene
c) 1-butene
d) 2-methyl-1-propanol
View Answer
Explanation: Reaction of a ketone with a phosphonium ylide results in a nucleophilic addition reaction to form a betaine which undergoes ring closure followed by elimination of a phosphine oxide to make a new carbon-carbon double bond of an alkene. This is a Wittig reaction. It is important to recognize that the new carbon-carbon double bond in the product is formed between the carbon atom of the carbonyl and the phosphorus-substituted carbon atom of the ylide. It is also useful to draw out the mechanism for this reaction to determine the structure of the product.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice basic questions and answers on all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
