This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Aromatic Amines”.
1. Aniline is usually purified by which of the following method?
a) Steam distillation
b) Simple distillation
c) Vacuum distillation
d) Extraction with a solvent
View Answer
Explanation: Aniline is usually purified by Steam distillation. The separation is long, tedious and potentially dangerous – involving steam distillation, solvent extraction and a final distillation.
2. Which of the following method cannot be used for preparation of aromatic amine?
a) Gabriel phthalimide synthesis
b) Reduction of nitro compound
c) Reduction of nitrile with LiAl4
d) Decarboxylation of amino acids
View Answer
Explanation: Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
3. What are A and B in the given sequence, respectively?
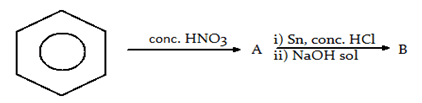
a) Aldehyde, nitro compound
b) Nitro compound, phenyl amine
c) Phenyl amine, nitro compound
d) Phenthalene, phenyl amine
View Answer
Explanation: Nitrobenzene is reduced to phenyl ammonium(A) ions using a mixture of tin and concentrated hydrochloric acid. The phenylamine(B) is formed together with a complicated mixture of tin compounds from reactions between the sodium hydroxide solution and the complex tin ions formed.
4. Which reducing agent is used for the reduction of nitro compound to phenyl amine?
a) LiAlH4
b) Sn/HCl
c) Na/alcohol
d) H2/Ni
View Answer
Explanation: Aromatic amines were prepared in good yields by a novel reduction of aromatic nitro compounds with tin metal in hydrochloric acid.
5. What is the known name of the reaction given below?
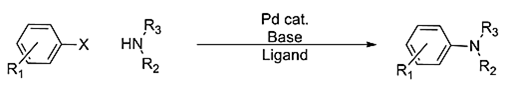
(where, X=Cl, Br, I, OTf; R2=Alkyl, aryl, H; R3=alkyl, aryl)
a) Gabriel phthalimide synthesis
b) Buchwald-Hartwig Reaction
c) Chan-Lam coupling
d) Ullmann reaction
View Answer
Explanation: The Buchwald–Hartwig amination is a chemical reaction used in organic chemistry for the synthesis of carbon–nitrogen bonds via the palladium-catalyzed cross-coupling of amines with aryl halides.
6. What is the known name of the reaction given below?

a) Gabriel phthalimide synthesis
b) Buchwald-Hartwig Reaction
c) Chan-Lam coupling
d) Ullmann reaction
View Answer
Explanation: The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides and copper and substituted ammonia to form aromatic amines. The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields.
7. What is the known name of the reaction given below?

a) Gabriel phthalimide synthesis
b) Buchwald-Hartwig Reaction
c) Chan-Lam coupling
d) Ullmann reaction
View Answer
Explanation: This reaction allows aryl carbon-heteroatom bond formation via an oxidative coupling of boronic acids, stannanes or siloxanes with N-H containing compounds in air. The reaction is induced by a stoichiometric amount of copper(II) or a catalytic amount of copper catalyst which is reoxidized by atmospheric oxygen or another primary oxidant.
8. Which type medium is required for the formation of aniline by reaction of aryl boric acid and HAS?
a) Acidic
b) Basic aqueous
c) Neutral dry
d) aqueous
View Answer
Explanation: Various anilines are prepared by treatment of functionalized aryl boronic acids with H2N-OSO3H (HSA) as a common, inexpensive source of electrophilic nitrogen, under basic aqueous conditions.
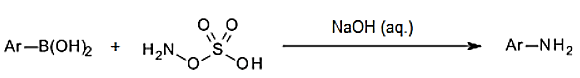
9. What will be the product for the following reaction?
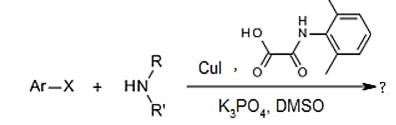
a) secondary aliphatic amine
b) primary aromatic amine
c) tertiary aromatic amine
d) secondary aromatic amine
View Answer
Explanation: this reaction is N- arylation of acyclic secondary amine, in which CuI and DMPAO are catalyst.
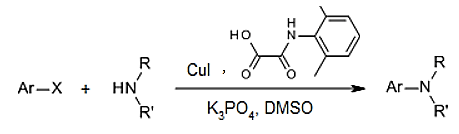
10. In the direct amination of alkyl and aryl pinacol boronates to form aromatic amine, which of the following is used as reagent with pinacol?
a) Aryl boric acid
b) Copper oxide
c) Pd(OAc)2
d) Lithiated methoxyamine
View Answer
Explanation: The direct amination of alkyl and aryl pinacol boronates with lithiated methoxyamine provides aliphatic and aromatic amines, stereospecifically, and without preactivation of the boronate substrate.
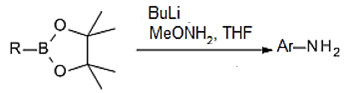
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Organic Chemistry Books
