This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Transesterification”.
1. Which of the following is true about transesterification?
a) exchanging the organic alkyl group of an ester with the organic group alkyl of an alcohol
b) exchanging the organic alkyl group of an alcohol with the organic group alkyl of an ester
c) exchanging the organic alkyl group of an ester with the organic group alkyl of an alkane
d) exchanging the organic alkyl group of an ester with the organic group alkyl of an ether
View Answer
Explanation: Transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol.
2. What is the geometry of the intermediate formed in the mechanism of transesterification?
a) Octahedral
b) Square planner
c) Tetrahedral
d) Square bipyramidal
View Answer
Explanation: In the transesterification mechanism, the carbonyl carbon of the starting ester reacts to give a tetrahedral intermediate, which either reverts to the starting material, or proceeds to the transesterified product.
3. What chemical reaction makes biodiesel?
a) Transesterification
b) Sublimation
c) Polymerization
d) Fermentation
View Answer
Explanation: Transesterification chemical reaction makes biodiesel. The most common method of transesterification is the reaction of the ester with an alcohol in the presence of an acid catalyst.
4. Polyethylene terephthalate is prepared by a transesterification reaction.
a) True
b) False
View Answer
Explanation: By transesterification reaction between dimethyl terephthalate and the dihydric alcohol, ethylene glycol, polyethylene terephthalate is prepared along with methanol, which is evaporated to drive the reaction forward.
5. What type of solution is used in transesterification for the determination of fatty acid compositions?
a) Methanol in NaOH
b) Methanol in NaCl
c) Methanol in KOH
d) Methanol in H2O
View Answer
Explanation: In aqueous warm solution of methanol in NaOH esterification for the determination of fatty acid compositions.
6. Transesterification reaction is an irreversible reaction.
a) True
b) False
View Answer
Explanation: The most common method of transesterification is the reaction of the ester with an alcohol in the presence of an acid catalyst. Since both the reactants and the products are an ester and an alcohol, the reaction is reversible and the equilibrium constant is close to one.
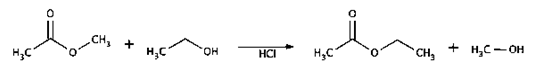
7. Which of the following is the product formed when methyl ethanoate and ethanol in presence of hydrochloric acid?
a) Ethyl ethanoate
b) Methyl ethanoate
c) Ethyl propanoate
d) Propyl ethanoate
View Answer
Explanation: Methyl ethanoate reacts with ethanol in the presence of hydrochloric acid to form ethyl ethanoate and methanol.
8. Which of the following is the product formed when alkylene carbonate and alcohol in presence of hydrochloric acid?
a) Dialkyl carbonate
b) Methyl ethanoate
c) Alkyl carbonate
d) Propyl ethanoate
View Answer
Explanation: The transesterification reaction implies an alkylene or cyclic carbonate and an alcohol in the presence of either a homogeneous or heterogeneous acidic or basic catalyst to coproduce dialkyl carbonate and the alkane diol or glycol.
9. Which of the following is the product formed when methyl propanoate and ethanol in presence of hydrochloric acid?
a) Ethyl ethanoate
b) Methyl ethanoate
c) Ethyl propanoate
d) Propyl ethanoate
View Answer
Explanation: Methyl propanoate reacts with ethanol in the presence of hydrochloric acid to form ethyl propanoate and methanol.
10. Which of the following is the product formed when butyl acetate and ethanol in presence of hydrochloric acid?
a) Ethyl ethanoate
b) Methyl acetate
c) Ethyl acetate
d) Propyl ethanoate
View Answer
Explanation: Butyl acetate reacts with ethanol in the presence of hydrochloric acid to form ethyl acetate and butanol.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
