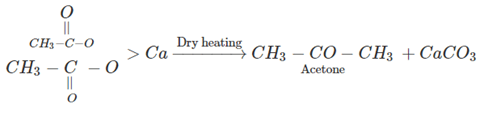This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Ketones”.
1. What is the end product in the following sequence of reaction?
![]()
a) Acetic acid
b) Isopropyl alcohol
c) Acetone
d) Ethanol
View Answer
Explanation: Alkyne will be converted into aldehyde and oxidation with Grignard reagent will for alcohol and then acetone will be formed.
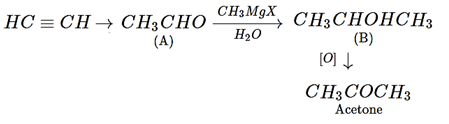
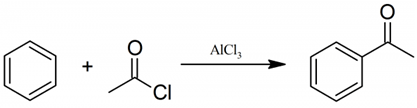
2. Acetophenone is prepared from which reaction?
a) Rosenmund reaction
b) Sandmayer reaction
c) Wurtz reaction
d) Friedel craft reaction
View Answer
Explanation: Acetophenone is prepared from Friedel craft reaction, in the presence of acetyl chloride, as shown below.
3. Compound which gives acetone on ozonolysis is?
a) CH3−CH=CH−CH3
b) (CH3)2C=C(CH3)2
c) C6H5CH=CH2
d) CH3CH=CH2
View Answer
4. Which one of the following compounds is prepared in the laboratory from benzene by a substitution reaction?
a) Glyoxal
b) Cyclohexane
c) Acetophenone
d) Hexabromo cyclohexane
View Answer
Explanation: Acetophenone is prepared in the laboratory from benzene by a substitution reaction.
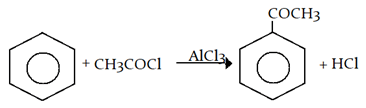
5. Ketones can be prepared in one step from which of the following process?
a) Hydrolysis of esters
b) Oxidation of primary alcohol
c) Oxidation of secondary alcohol
d) Reaction of acid halide with alcohols
View Answer
Explanation: Secondary alcohols are oxidized to ketones – and that’s it. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate(VI) solution acidified with dilute sulfuric acid, propanone is formed. Changing the reaction conditions makes no difference to the product.
6. Ketones are prepared by which of the following name reaction?
a) Clemmensen’s reduction
b) Cannizzaro reaction
c) Rosenmund’s reduction
d) Oppenaur’s oxidation
View Answer
Explanation: Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.
7. Isopropyl alcohol on oxidation gives which of the following?
a) Acetone
b) Acetaldehyde
c) Ether
d) Ethylene
View Answer
Explanation: Isopropyl alcohol can be oxidized to acetone, which is the corresponding ketone. This can be achieved using oxidizing agents such as chromic acid, or by dehydrogenation of isopropyl alcohol over a heated copper catalyst.
8. Dry heating of calcium acetate gives which of the following?
a) Acetaldehyde
b) Ethane
c) Acetic acid
d) Acetone
View Answer
9. Which of the following compound gives a ketone with Grignard reagent?
a) Formaldehyde
b) Ethyl alcohol
c) Methyl cyanide
d) Methyl iodide
View Answer
Explanation: Methyl cyanide gives a ketone with Grignard reagent, as shown in below reaction.
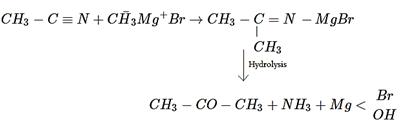
10. Propyne on hydrolysis in presence of HCl and HgSO4 gives which of the following?
a) Acetaldehyde
b) Acetone
c) Formaldehyde
d) Acetophenone
View Answer
Explanation: Alkynes react with water in the presence of HgSO4 to give an alcohol with double bond. This alcohol is called as enol (en for double bond and ol for alcohol). These enols are highly unstable and undergo migration to give ketone.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books