This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Saponification”.
1. Which of the following processes can saponification be used for?
a) For the production of plastics
b) In blow glass artistry
c) To make soap
d) The formation of alloys
View Answer
Explanation: Saponification is a process that produces soap. Soaps are salts of fatty acids.
2. What is the name of the soap produced through the saponification of this triglyceride?
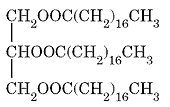
a) Sodium dececanoate
b) Sodium oleate
c) Sodium stearate
d) Sodium acetate
View Answer
Explanation: The soap has the formula C17H35COONa and its name is sodium stearate.
3. Lye is used in soap-making. Lye is a concentrated solution of which ionic compound?
a) K2CO3
b) CuSO4
c) NaOH
d) NaCl
View Answer
Explanation: Lye is another name for a strongly basic solution. Mostly it is a solution of sodium hydroxide.
4. Which of the following term describe saponification?
a) Cleaving of ester molecules into carboxylic acid and alcohol
b) Dehydration synthesis by removing water
c) Hydrolysis of a salt by adding a weak acid
d) Synthesis of two alkyl groups to make an ether
View Answer
Explanation: Cleaving of ester molecules into carboxylic acid and alcohol that is triglyceride is treated with a strong base, which cleaves the ester bond, releasing fatty acid salts (soaps) and glycerol.
5. Which of the following is considered a useful alkali in saponification reactions?
a) CCl4
b) Cl–
c) NaOH
d) Pb+
View Answer
Explanation: Depending on the nature of the alkali used in their production, soaps have distinct properties. Sodium hydroxide (NaOH) gives “hard soap”; hard soaps can also be used in water containing Mg, Cl, and Ca salts.
6. Which of the following statement is true about saponification value of oil?
a) The shorter the chain of fatty acid, the higher is the saponification value
b) The shorter the chain of fatty acid, the higher is the saponification value
c) The higher the saturation in chain of fatty acid, the lower is the saponification value
d) The lower the saturation in chain of fatty acid, the higher is the saponification value
View Answer
Explanation: The shorter the chain of fatty acid, the higher is the saponification value, the long chain fatty acids found in fats have a low saponification value because they have a relatively fewer number of carboxylic functional groups per unit mass of the fat as compared to short chain fatty acids.
7. Which of the following fat or oil is unsaponifiable?
a) Paraffin wax
b) Bee wax
c) Olive oil
d) Shea butter
View Answer
Explanation: Unsaponifiables are components of an oily (oil, fat, wax) mixture that fail to form soaps when treated with sodium hydroxide (lye) or potassium hydroxide. Unsaponifiable value of paraffin wax is approximately 100.
8. Saponification value is the number of milligrams of KOH required to saponify what present in the 1g of oil or fat?
a) Salts
b) Hydrocarbon
c) Fatty acids
d) unsaturation
View Answer
Explanation: The saponification is the value of oil determined as the number of mgs of KOH needed to saponify the fatty acids present in the 1g of oil.
9. Which of the following compound is an industrial manufacturing product by saponification?
a) Sodium chloride
b) Potassium hydroxide
c) Glycerol
d) Sodium hydroxide
View Answer
Explanation: The triglyceride is treated with a strong base which cleaves the ester bond, releasing fatty acid salts (soaps) and glycerol. This process is also the main industrial method for producing glycerol. In some soap-making, the glycerol is left in the soap.
10. Soap can be precipitated out by salting by using which chemical compound?
a) Sodium chloride
b) Potassium hydroxide
c) Glycerol
d) Sodium hydroxide
View Answer
Explanation: If necessary, soaps may be precipitated by salting it out with sodium chloride.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
