This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Reactions of Glycols”.
1. Which catalyst is used for reaction of ethylene glycol with acetic acid?
a) Amberlyst 36
b) Hydrogen peroxide
c) Potassium permanganate
d) Aluminium bromide
View Answer
Explanation: Esterification of ethylene glycol with acetic acid to ethylene glycol monoacetate and ethylene glycol diacetate using the acidic ion-exchange resin Amberlyst 36 as catalyst were investigated.
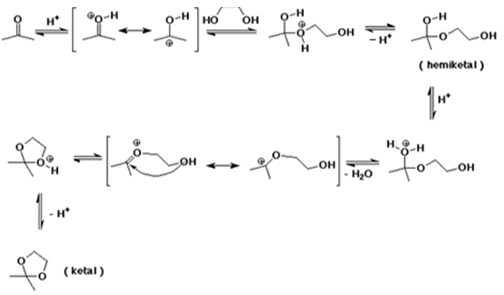
2. What is the principle product of the acid catalysed reaction of acetone and ethylene glycol?
a) 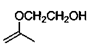
b)![]()
c) ![]()
d) 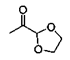
View Answer
Explanation: According to the given reaction between acetone and glycol, we can show that cyclic ketal is the product.
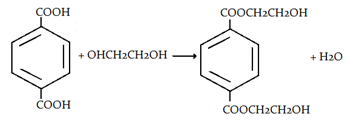
3. What will be the product when ethylene glycol and terephthalic acid reacts?
a) 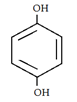
b) 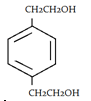
c) 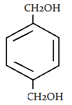
d) 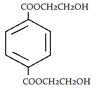
View Answer
4. What happens when glycol reacts with lead tetraacetate?
a) No reaction
b) Ketones will be formed
c) Aldehyde will not be formed
d) Monohydric alcohols will be formed
View Answer
Explanation: Lead tetraacetate is used cleave a carbon-carbon bond in a glycol. This reaction is useful in the formation of ketones and aldehydes and involves a favourable five membered cyclic intermediate.
5. What is the intermediate form in the mechanism of the lead acetate and glycol?
a) Three membered cyclic ring
b) Four membered cyclic ring
c) Five membered cyclic ring
d) Six membered cyclic ring
View Answer
Explanation: Lead tetraacetate is used cleave a carbon-carbon bond in a glycol and involves a favourable five membered cyclic intermediate, as shown in given reaction.
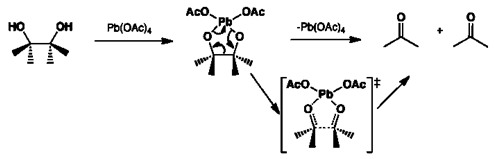
6. What is the name of the reaction when lead tetraacetate is used cleave a carbon-carbon bond in a glycol?
a) Criegee oxidation
b) Swern oxidation
c) Baeyer-Villiger Oxidation
d) Jones oxidation
View Answer
Explanation: The oxidation of 1,2‐diols (or glycols) to aldehydes or ketones with lead tetraacetate [Pb(OAc)4, LTA] via the cleavage of C‐C bond between the hydroxyl‐carrying carbon atoms is generally known as Criegee Glycol oxidation. This reaction has been reported to occur only in anhydrous organic solvent.
7. Which of the following statement is not true about criegee oxidation?
a) The oxidative cleavage of an alpha,beta-diol using lead tetraacetate to give the corresponding carbonyl compounds
b) It is analogous but milder than the Malaprade reaction
c) This oxidation was discovered by Rudolf Criegee and coworkers and first reported in 1931
d) Rate of reaction do not depend upon stereochemistry of glycol
View Answer
Explanation: The rate of the reaction is highly dependent on the conformation of the diols, so much so that diols that are cis on certain rings can be reacted selectively as opposed to those that are trans on them.
8. Criegee oxidation of glycol should be performed in which type of medium?
a) Hydrous
b) Anhydrous
c) Acidic
d) Basic
View Answer
Explanation: It was heavily stressed by Criegee that the reaction must be run in anhydrous solvents, as any water present would hydrolyze the lead tetraacetate; however, subsequent publications have shown that if the oxidation is faster than the rate of hydrolysis, the cleavage can be run in wet solvents or even aqueous solutions.
9. What happens when glycol reacts with periodic acid?
a) No reaction
b) Ketones will be formed
c) Aldehyde will not be formed
d) Monohydric alcohols will be formed
View Answer
Explanation: Oxidation with Periodic Acid is used to cleave vicinal diols (a total of two alcohols, one on two adjacent carbons) into two carbonyl molecules.
10. Ethylene glycol on treatment with phosphorus tri-iodide yields
a) ethyl iodide
b) ethylene di-iodide
c) ethylene
d) ethane
View Answer
Explanation: Phosphorus triiodide (PI3) is an unstable red solid which reacts violently with water. It is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Check Chemical Engineering Books