This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Reaction Intermediates”.
1. Which carbocation is the most stable?
a) 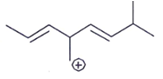
b) 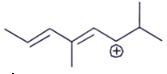
c) 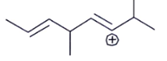
d)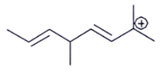
View Answer
Explanation: This is stabilized by extended conjugation. The more adjacent methyl groups there are, the larger hyperconjugation stabilization is because of the increased number of adjacent C–H bonds.
2. Which one among the following carbocations has the longest half-life?
a) 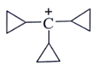
b) 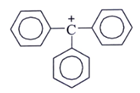
c) 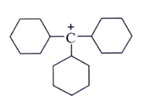
d) 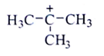
View Answer
Explanation: Higher the stability of cation more will be its half-life. As, in the carbocation, p orbital overlap with the compound, will be more stabilizing factor than aromaticity.
3. The order of decreasing stability of the following cations is?
(I) CH3C+HCH3 (II) CH3C+HOCH3 (III) CH3C+HCOCH3
a) III > II > I
b) I > II > III
c) II > I > III
d) I > III > II
View Answer
Explanation: (II) Here, Mesomeric effect stabilizes carbocation. So, it is highly stabilized.
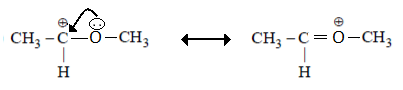
(I) It is stabilized by hyperconjugation.
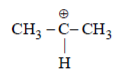
(III) It is destabilized as because of carbonyl group; more electronegative Oxygen is present it will decrease electron density of C+.
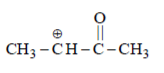
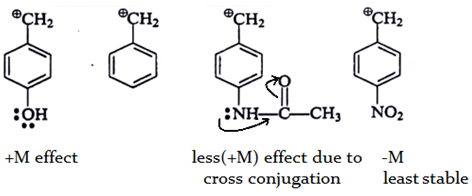
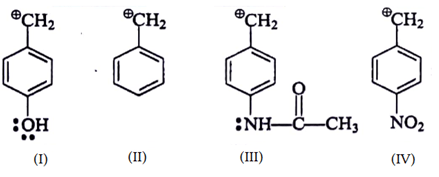
4. Which of the following is most stable intermediate?
a) 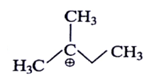
b) 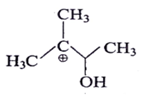
c) 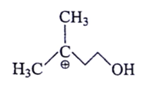
d) 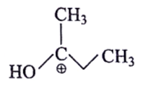
View Answer
Explanation: Stabilized by +M effect of OH and hyperconjugation effect (5αH).
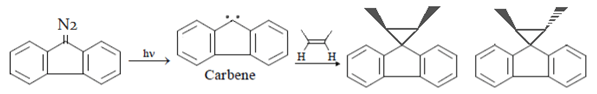
5. Which intermediate is involved in the reaction given below?
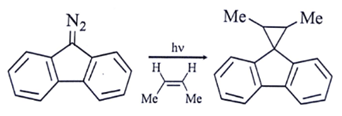
a) free radical
b) carbocation
c) carbanion
d) carbene
View Answer
6. Among the following which is most stabilized cation?
a) 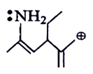
b) 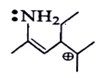
c) 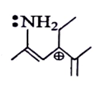
d) 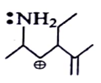
View Answer
Explanation: The positive charge of carbon will be stabalised by mesomeric effect of -NH2 group.
7. Arrange the following intermediate into decreasing order of stability.
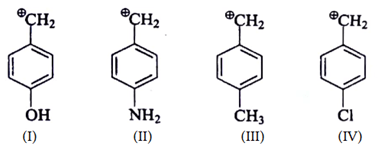
a) I > II > III > IV
b) II > IV > III > I
c) II > I > IV > III
d) II > I > III > IV
View Answer
Explanation: Mesomeric effect will stabilize carbocation is highly stable.
+M effect of –NH2 > –OH
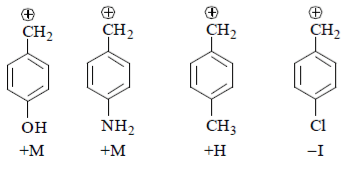
8. Arrange the following intermediate into decreasing order of stability.
a) I > III > II > IV
b) II > III > IV > I
c) III > IV > II > I
d) I > III > IV > II
View Answer
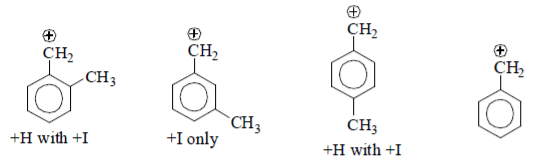
9. What is the correct decreasing order of stability of following cation?
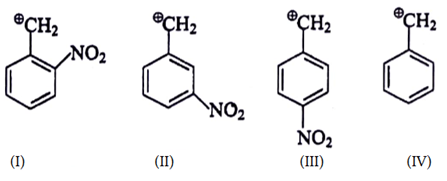
a) II > III > I > IV
b) IV > II > III > I
c) IV > III > I > II
d) III > I > II > IV
View Answer
Explanation:
(I) It is destabilized by –M and high –I effect.
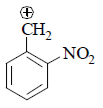
(II) It is destabilized by –I nature only at meta position –M effect.
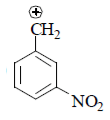
(III) It is destabilized by –M and less –I effect.
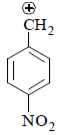
(IV) There is no destabilisation by group.
10. What is the correct decreasing order of stability of following cation?
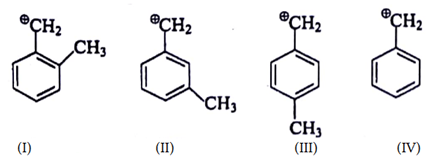
a) I > II > III > IV
b) I > IV > II > III
c) I > III > II > IV
d) IV > III > II > I
View Answer
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs