This set of Organic Chemistry Questions and Answers for Campus interviews focuses on “Alcohols as Acids”.
1. CH3OH is a which type of acid?
a) Bronsted acid
b) Lewis acid
c) Arrhenius acid
d) Lewis and Arrhenius acid
View Answer
Explanation: Alcohols are very weak Bronsted acids with pKa values generally in the range of 15 – 20. Because the hydroxyl proton is the most electrophilic site, proton transfer is the most important reaction to consider with nucleophiles.
2. Which of the following is more acidic than alcohols?
a) Arrhenius acid
b) amine
c) alkyne
d) carboxylic acid
View Answer
Explanation: As there will be resonance between -COO–. So, it will be more stable and acidic than -OH.
3. What is the correct order of acidic strength?
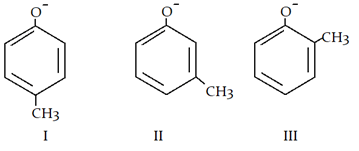
a) I > II > III
b) II > I > III
c) III > II > I
d) I > III > II
View Answer
Explanation: Inductive effect (+I) destabilises phenol ion and III has maximum +I effect and in II -CH3 is at meta position so no effect on phenol ion negative charge.
4. Which of the following is more acidic alcohol?
a) Phenol
b) Cyclohexanol
c) Methanol
d) Ethanol
View Answer
Explanation: Resonance stabalizes the negative charge in phenol ion, negative charge is moving throughout. So, phenol is more acidic.
5. What is the correct order of acidic strength?
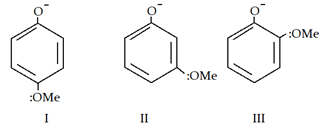
a) I > II > III
b) III > II > I
c) II > III > I
d) I > III > II
View Answer
Explanation: Inductive effect (-I) stabilises phenol ion and II has maximum -I effect. But at para and ortho position +M effect will also show effect so they are les acidic than meta.
6. What is the correct order of acidic strength?
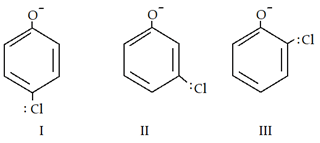
a) I > II > III
b) III > II > I
c) II > III > I
d) I > III > II
View Answer
Explanation: Inductive effect (-I) stabilises phenol ion and II has maximum -I effect. Less the distance between electron withdrawing group and negative charge more the stability and acidity too.
7. What is the correct order of acidic strength?
a) CH3-OH
b) CH3CH2-OH
c) CH3 CH2 CH2-OH
d) CH3 CH2 CH2 CH2-OH
View Answer
Explanation: Inductive effect (+I) destabilises -OH ion, so CH3-OH is most acidic alcohol among above, as it has least +I.
8. What is the correct order of acidic strength?
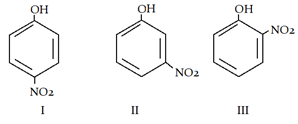
a) I > II > III
b) III > II > I
c) II > III > I
d) I > III > II
View Answer
Explanation: Electron withdrawing group stabalises phenol ion. More the -M effect, more the stability.
9. What is the correct order of acidic strength?
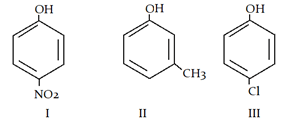
a) I > II > III
b) III > II > I
c) II > III > I
d) I > III > II
View Answer
Explanation: Electron withdrawing group stabalises phenol ion and electron donating group destablises it. More the -M effect, more the stability, so I is most stable and acidic.
10. What is the correct order of acidic strength?

a) I > II > III
b) III > II > I
c) II > I > III
d) I > III > II
View Answer
Explanation: Electron withdrawing group stabalises phenol ion. More the -M effect, more the stability.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry for Campus Interviews, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
- Check Chemical Engineering Books
- Check Organic Chemistry Books
