This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Preparation of Phenols”.
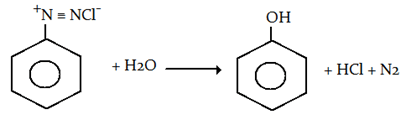
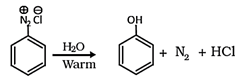
1. On heating aqueous solution of benzene diazonium chloride, which of the following is formed?
a) benzene
b) chlorobenzene
c) phenol
d) aniline
View Answer
2. Sodium benzene sulphonate reacts with NaOH and then on acidic hydrolysis, it gives which of the following compound?
a) Phenol
b) Benzoic acid
c) Benzene
d) Disodium benzaldehyde
View Answer
Explanation: Sodium benzene sulphonate reacts with NaOH and then on acidic hydrolysis, phenol.
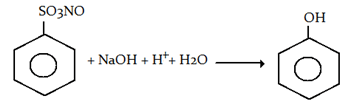
3. What is the commercial method of preparation of phenol?
a) Dows process
b) From diazonium salt
c) By decarboxylation of salicylic acid
d) Hock method
View Answer
Explanation: The Hock process (cumene-phenol process, cumene process) is an industrial process for developing phenol and acetone from benzene and propylene. The term stems from cumene (isopropyl benzene), the intermediate material during the process.
4. Reaction of aqueous sodium hydroxide on chlorobenzene gives which of the following products?
a) o-chlorophenol
b) o-chlorophenol
c) phenol
d) no reaction
View Answer
Explanation: Chlorobenzene does not undergo hydrolysis under normal conditions. However, it undergoes hydrolysis when heated in an aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm to form phenol.
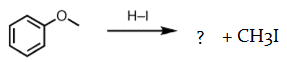
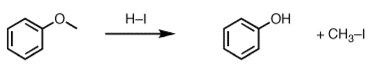
5. What will be the product ‘a’ for the given reaction?
a)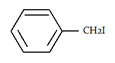
b) 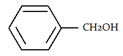
c) 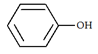
d) 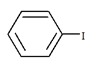
View Answer
Explanation: When ethers are treated with strong acid in the presence of a nucleophile, they can be cleaved to give alcohols and alkyl halides.
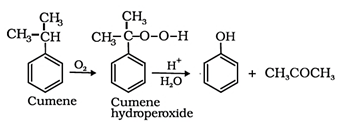
6. What is the reagent which will react with cumene to give phenol?
a) Oxygen
b) Hydrogen
c) Nitrogen
d) Ozone
View Answer
Explanation: Upon oxidation of cumene (isopropylbenzene) in presence of air (oxygen), cumene hydroperoxide is obtained. Upon further treatment of cumene hydroperoxide with dilute acid phenols are obtained.
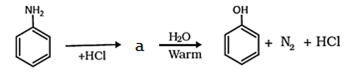
7. What will be the product ‘a’ in the given reation?
a) Enolate form
b) Benzene diazonium chloride
c) Benzene
d) Chlorobenzene
View Answer
Explanation: When an aromatic primary amine is treated with nitrous (NaNO2 + HCl) acid at 273 – 278 K, diazonium salts are obtained. Upon warming with water, these diazonium salts finally hydrolyze to phenols.
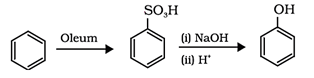
8. What is the reactant ‘x’ and ‘y’ that will react with benzene to give phenol?
a) X is Oleum and y is molten sodium hydroxide followed by H+
b) X is Oleum and y is HCl
c) X is Oleum and y is NH3
d) X is Oleum and y is water
View Answer
Explanation: Benzenesulphonic acid can be obtained from benzene by reacting it with oleum. Benzenesulphonic acid thus formed is treated with molten sodium hydroxide at high temperature which leads to the formation of sodium phenoxide. Finally, sodium phenoxide on acidification gives phenols.
9. Which of the following is not a method for preparation of phenol?
a) Dows process
b) From diazonium salt
c) By decarboxylation of salicylic acid
d) By the decarboxylation of sodium benzoate
View Answer
Explanation: The decarboxylation of sodium benzoate is not the method of preparation of phenol, this method is used for the preparation of benezene.
10. Phenol is obtained by heating aqueous solution of which of the following?
a) Aniline
b) Benzene diazonium chloride
c) Benzoic acid
d) Benzyl alcohol
View Answer
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books