This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Reactions of Glycerol”.
1. What will be the product for the reaction of catalytic hydrogenation of glycerol?
a) Glycerol carbonate
b) Epichlorohydrine
c) Propylene glycol
d) Ethylene glycol
View Answer
Explanation: Glycerol is easily reduced to propylene glycol (1,2- dihydroxypropane) with hydrogen at pressures from 10 to 100 atmospheres and temperatures above 150° C. Many catalysts may be used, e.g. Ni, Fe, Pt, Au, Hg, copper chromite or tungstic acid.
2. What will be the product for when glycerol is heated with hydriodic acid?
a) Glycerol carbonate
b) Epichlorohydrine
c) Ethylene glycol
d) Isopropyl iodide
View Answer
Explanation: Glycerol heated to 135 to 140℃ with an excess of hydriodic acid is reduced to isopropyl iodide. This reaction is the basis of the Zeisel-Fanto analytical method for determining glycerol.
3. What will be product of the reaction if lead tetraacetate and glycerol will react?
a) Glycerol carbonate
b) Ethylene glycol
c) Formic acid
d) Formaldehyde and formic acid
View Answer
Explanation: Lead tetraacetate, like periodic acid, will oxidize polyhydric alcohols with adjacent hydroxyl groups. Two moles of formaldehyde and one mole of formic acid are formed from one mole of glycerol.
4. What will be product of the following reaction?
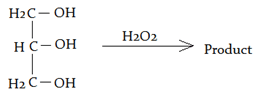
a) Glycolic acids
b) Glyceric acid
c) Formic acid
d) Formaldehyde
View Answer
Explanation: When glycerol is distilled with hydrogen peroxide which is added intermittently, it is quantitatively converted into formic acid while glyceric and glycolic acids are formed as intermediate products.
5. What will be the product for the following reaction?
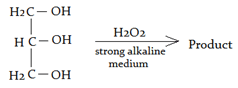
a) Glycolic acids
b) Glyceric acid
c) Formic acid
d) Formaldehyde
View Answer
Explanation: Glycerol oxidized by hydrogen peroxide in a strongly alkaline solution results in the formation of formaldehyde and the production of hydrogen, but neither is formed when the oxidation takes place in a less alkaline medium.
6. What will happen when glycerol is added into bromine water and sodium carbonate?
a) Glycerol carbonate
b) Dihydroxyacetone
c) Ethylene glycol
d) Isopropyl iodide
View Answer
Explanation: Dihydroxyacetone results from the oxidation of glycerol with bromine and sodium carbonate and by the oxidation of lead glyceroxide with bromine vapors.
7. What will happen when glycerol is added into dimethyl carbonate?
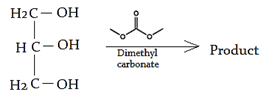
a) Glycerol carbonate
b) Dihydroxyacetone
c) Glycerol formate
d) Isopropyl iodide
View Answer
Explanation: Glycerol carbonate prepared by heating such esters as dimethyl carbonate with glycerol, or reacting glycerol with phosgene in the presence of organic bases such as pyridine, triethylamine or quinoline.
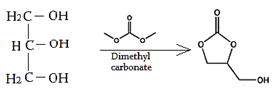
8. What will happen when glycerol is added into dimethyl carbonate?

a) Glycerol carbonate
b) Dihydroxyacetone
c) Glycerol formate
d) Isopropyl iodide
View Answer
Explanation: Glycerol carbonate prepared by heating such esters as urea with glycerol, or reacting glycerol with phosgene in the presence of organic bases such as pyridine, triethylamine or quinoline.

9. What will happen when glycerol is added into dimethyloxalate?
a) Glycerol carbonate
b) Dihydroxyacetone
c) Glycerol formate
d) Glycerol oxalate
View Answer
Explanation: Glycerol reacts with methyl oxalate to produce glycerol oxalate, which decomposes at 220-225℃ to form ally1 alcohol, carbon monoxide, carbon dioxide and an oil.
10. What will happen when glycerol is added into acid?
a) Esterification
b) Alcoholysis
c) Transesterification
d) No reaction will occur
View Answer
Explanation: When glycerol is added into acid esterification will occurs, as shown in given reaction.
C3 H5 (OH)3+RCOOH → C3 H5 (OH)2 OOCR+ H2O
Glycerol Acid Ester Water
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
