This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Allyl & Vinylic Halides”.
1. Which of the following is not an allylic halide?
a) ![]()
b) 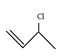
c) 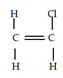
d) 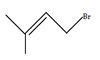
View Answer
Explanation: When a halogen (F, Cl, Br) is attached to a sp3 carbon of a propene, it is called an allylic halide. The propene in turn can be attached to other elements. An allylic halide is an alkyl halide which has one or more halide at allylic carbon.
2. Which of the following statement is incorrect about allylic halide?
a) The positions adjacent to alkene C=C often show decrement in reactivity
b) SN1 reactions of allylic halides are possible
c) Allylic bonds are often weaker and therefore more easily broken
d) stability of the allylic radical can be utilized in the preparation of allylic halides
View Answer
Explanation: The positions adjacent to alkene C=C often show enhanced reactivity compared to simple alkanes due to the proximity of the adjacent π system. Such positions are referred as “allylic”.
3. How many resonating structure of allylic carbocation is present?
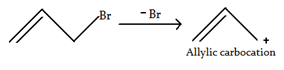
a) 1
b) 2
c) 3
d) 4
View Answer
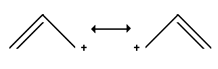
Explanation: There are two resonation structures, as shown below. In the two resonance forms of the allylic cation, the positive charge is located on the terminal carbon atoms and never on the middle carbon.
4. Which of the following is considered as allylic carbon atom?
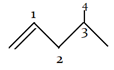
a) 1
b) 3
c) 2
d) 4
View Answer
Explanation: An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
5. Which of the following allylic halide has commercial usage?
a) H2C = CH−CH2Cl
b) H2C = CH−CH2Br
c) H2C = CH−CH2I
d) H2C = CH−CH2F
View Answer
Explanation: As an alkylating agent, allylic chloride is useful in the manufacture of pharmaceuticals and pesticides, such as mustard oil.
6. What is the desired product for the following reaction?
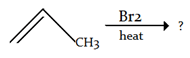
a) 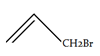
b) 
c) 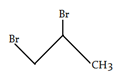
d) 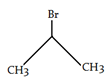
View Answer
Explanation: We have 1 equivalent of Br2 swimming around, of which only a small proportion at any given time will exist as bromine radical [due to the initiation step]. we had a very low concentration of Br2. If the concentration of Br2 is kept low, not only will the rate of dibromination be lower, the relative rate of bromine radical relative to Br2 will increase. Therefore, the rate of C-H abstraction relative to dibromination will increase, which will allow our allylic bromination product to be formed in a higher yield.
7. Which of the following is a vinylic halide?
a) ![]()
b) 
c) 
d) 
View Answer
Explanation: In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.
8. Which of the following vinyl halide has industrial application?
a) Vinyl fluoride
b) Vinyl chloride
c) Vinyl bromide
d) Vinyl iodide
View Answer
Explanation: Vinyl chloride is an organohalogen compound that has important industrial applications. When treated with certain catalysts, vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride, or PVC. PVC is used in the manufacture of numerous products, including packaging films and water pipes.
9. Which of the following is the product for the given reaction?

a) (CH3)3CCH≡CH
b) (CH3)3CCH=CH2
c) (CH3)3CCH=CHOH
d) (CH3)3CCH=CHNH2
View Answer
Explanation: Vinyl halides undergo elimination reactions similar to alkyl halides, although at slower rates, and they normally require very strong bases such as sodium amide (NaNH2).
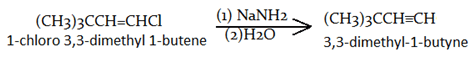
10. How can be a vinylic halide converted into Grignard reagent?
a) Addition of base
b) Addition of acid
c) By adding magnesium
d) Addition of diethyl ether
View Answer
Explanation: Vinylic halides may be converted to Grignard reagents by reaction with magnesium, as shown below:
11. What will be the product when addition of hydrogen chloride to vinyl chloride occurs?
a) 1,1-dichloroethane
b) 1,2-dichloroethane
c) 1-chloroethane
d) 1-chloro 2,2-dichloroethane
View Answer
Explanation: The addition of hydrogen chloride to vinyl chloride to yield 1,1-dichloroethane. The product is a geminal dihalide (both halogens are bonded to the same carbon).
12. What will be the product for the given reaction?
CH2 = CHC ≡ CH + HCl → ?
vinylacetylene
a) 2-chloro-1,3-dibutadiene
b) 1,2-dichloro-1,3-dibutadiene
c) 1-chloro-2,3-dibutadiene
d) 3-chloro-1,2-dibutadiene
View Answer
Explanation: 2-chloro-1,3-dibutadiene the monomer used in the formation of the elastomer neoprene, is prepared from vinylacetylene by this reaction.
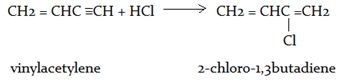
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Check Organic Chemistry Books
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
