This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Esters”.
1. Which of the following compounds is an ester?
a) 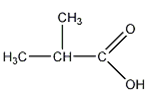
b) 
c) 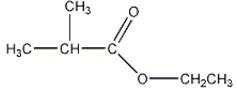
d) 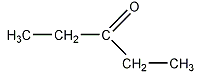
View Answer
Explanation: Esters are characterized by their possession of a modified carboxyl group, in which the H of the -OH group is replaced by an alkyl group, to form the general functional group -COOR.
2. Which of the following is used as catalyst for the esterification of carboxylic acid and alcohol?
a) Nitrous acid
b) Sulphuric acid
c) Sulphurous acid
d) Nitric acid
View Answer
Explanation: The classic synthesis is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent:
RCO2H + R′OH ⇌ RCO2R′ + H2O
The equilibrium constant for such reactions is about 5 for typical esters, e.g., ethyl acetate. The reaction is slow in the absence of a catalyst. Sulfuric acid is a typical catalyst for this reaction.
3. What will be the product for the given reaction?
CH3OH + CO →?
a) Ethyl formate
b) Methyl formate
c) Ethyl acetate
d) Methyl acetate
View Answer
Explanation: The carbonylation of methanol yields methyl formate, which is the main commercial source of formic acid.
CH3OH + CO → CH3O2CH
4. Hydrolysis of ester leads to the formation of which of the following products in basic medium?
a) Ether and alcohol
b) Alcohol and sodium carboxylate
c) Aldehyde and alcohol
d) Sodium carboxylate
View Answer
Explanation: Under basic conditions, hydroxide acts as a nucleophile, while an alkoxide is the leaving group.
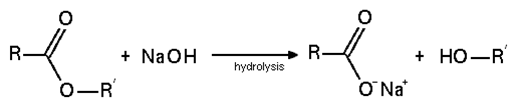
5. What will be the product for the given reaction?
C2H4 + CH3CO2H + 1⁄2 O2 →?
a) Ethyl formate
b) Vinyl formate
c) Ethyl acetate
d) Methyl acetate
View Answer
Explanation: In the presence of palladium-based catalysts, ethylene, acetic acid, and oxygen react to give vinyl acetate:
C2H4 + CH3CO2H + 1⁄2 O2 → C2H3O2CCH3 + H2O
6. What is the characteristic smell for ester?
a) Fruity like smell
b) Fish like smell
c) Rotten egg smell
d) Alcoholic smell
View Answer
Explanation: Many esters have distinctive fruit-like odors, and many occur naturally in the essential oils of plants. This has also led to their commonplace use in artificial flavorings and fragrances when those odors aim to be mimicked.
7. Salts of carboxylate anions can be alkylating agent with alkyl halides to give esters. In the case that an alkyl chloride is used, an iodide salt can catalyze the reaction. What is the name reaction for the given reaction?
a) Favorskii rearrangement
b) Baeyer–Villiger oxidation
c) Pinner reaction
d) Finkelstein reaction
View Answer
Explanation: Salts of carboxylate anions can be alkylating agent with alkyl halides to give esters. In the case that an alkyl chloride is used, an iodide salt can catalyze the reaction this reaction is known as Finkelstien reaction.
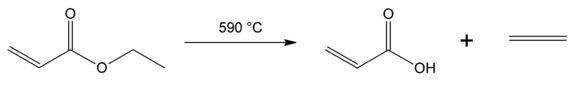
8. What will be the product of given reaction?

a) Ether and alcohol
b) Alcohol and sodium carboxylate
c) Aldehyde and alcohol
d) Carboxylic acid and alkene
View Answer
Explanation: Ester pyrolysis in is a vacuum pyrolysis reaction converting esters containing a β-hydrogen atom into the corresponding carboxylic acid and the alkene.
9. Fries rearrangement reaction of phenol ester leads to the formation which type of product?
a) Ketone
b) Aldehyde
c) Alcohol
d) Alkene
View Answer
Explanation: The Fries rearrangement is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
10. What is the order or solubility in water of the following compound?
i) ethyl methanoate ii) ethyl butanoate iii) ethyl ethanoate iv) ethyl propanoate
a) i > ii > iii > iv
b) i > iii > iv > ii
c) i > ii > iv > iii
d) iii > i > iv > ii
View Answer
Explanation: As chain length increases, the hydrocarbon portion forces itself between water molecules, breaking the relatively strong hydrogen bonds between water molecules without offering an energetic compensation; furthermore, the water molecules are forced into an ordered alignment along the chain, decreasing the entropy in the system. This makes the process thermodynamically less favorable, and so solubility decreases.
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Check Organic Chemistry Books
- Apply for Chemical Engineering Internship
