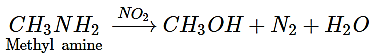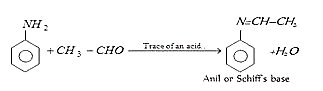This set of Organic Chemistry Multiple Choice Questions & Answers (MCQs) focuses on “Chemical Properties of Amines – 1”.
1. Which of the following is converted into an alcohol on treatment with HNO2?
a) Methyl amine
b) Aniline
c) Dimethyl amine
d) Triethyl amine
View Answer
2. The action of nitrous acid on ethyl amine gives which of the following?
a) Ethane
b) Ammonia
c) Ethyl acohol
d) Nitroethane
View Answer
Explanation: The action of nitrous acid on ethyl amine gives ethyl alcohol, along with nitrogen and water.
CH3CH2NH2 + HNO2 → CH3CH2OH + N2 + H2O
3. Indicate which nitrogen compound amongst the following would undergo Hofmann’s reaction (i.e. reaction with Br2 and strong KOH) to furnish the primary amine.
a) 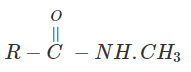
b) 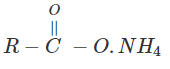
c) 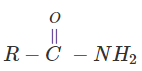
d) 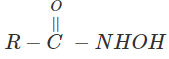
View Answer
Explanation: This reaction is Hoffman degradation of amide.
R – CONH2 + Br2 + 4KOH → R – NH2 +2KBr + k2CO3 + 2H2O.
4. Aniline reacts with acetaldehyde to form which of the following?
a) Schiff’s base
b) Carbylamine
c) Immine
d) Diazonium salt
View Answer
5. p-chloroaniline and anilinium hydrochloride can be distinguished by which test?
a) Sandmeyer reaction
b) NaHCO3
c) AgNO3
d) Carbylamine test
View Answer
Explanation: Anilinium hydrochloride is an acid salt and liberates CO2 from
NaHCO3. But p-chloro aniline is basic not acidic it does not liberate CO2. p-chloro aniline does not contain ionic chlorine to it does not give white ppt with AgNO3.
6. Nitroso amines (R2N−N=O) are soluble in water. On heating them with concentrated H2SO4 they give secondary amines. What is this reaction called as?
a) Perkin’s reaction
b) Fittig’s reaction
c) Sandmeyer’s reaction
d) Liebermann’s nitroso reaction
View Answer
Explanation: Nitroso amines (R2N−N=O) are soluble in water. On heating them with concentrated H2SO4 they give secondary amines, this reaction is called as Liebermann’s nitroso reaction.
7. Ethyl amine undergoes oxidation in the presence of KMnO4 to form which compound?
a) An acid
b) An alcohol
c) An aldehyde
d) A nitrogen oxide
View Answer
Explanation: Ethyl amine undergoes oxidation in the presence of KMnO4 to form an aldehyde.
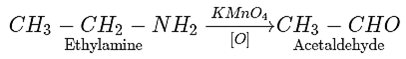
8. Reaction of primary amines with aldehyde yields which of the following compound?
a) Amides
b) Aldimines
c) Nitriles
d) Nitro compounds
View Answer
Explanation: Reaction of primary amines with aldehyde yields aldimines.
R – CH2 – NH2 + O = CH – R → R – CH2 – N = CH – R + H2O
1oamine aldehyde Aldimine
9. When primary amines are treated with HCl, the product obtained is which of the following?
a) An alcohol
b) A cyanide
c) An amide
d) Ammonium salt
View Answer
Explanation: When primary amines are treated with HCl, the product obtained is ammonium salt.
CH3 – CH2 – NH2 + HCl → CH3CH2 – NH3+Cl–
10. Primary amines can be distinguished from secondary and tertiary amines by reacting with which of the following?
a) Chloroform and alcoholic KOH
b) Methyl iodide
c) Chloroform alone
d) Zinc dust
View Answer
Explanation: Primary amine reacts with CHCl3 and alc. KOH to form isocyanide while secondary and tertiary amines do not react.
11. When chloroform reacts with ethyl amine in presence of alcoholic KOH, the compound formed is which of the following?
a) Ethyl cyanide
b) Ethyl isocyanide
c) Formic acid
d) An amide
View Answer
Explanation: When chloroform reacts with ethyl amine in presence of alcoholic KOH, the compound formed is ethyl isocyanide.
12. The compound which on reaction with aqueous nitrous acid on HNO2 at low temperature produces an oily nitrosoamine is which of the following?
a) Diethylamine
b) Ethylamine
c) Aniline
d) Methylamine
View Answer
Explanation: The compound which on reaction with aqueous nitrous acid on HNO2 at low temperature produces an oily nitrosoamine is diethylamine.
(C2H5)2NH + (aq.)HONO → (C2H5)2N – N = O + H2O
13. Aniline on treatment with excess of bromine water which of the following?
a) Aniline bromide
b) o-bromoaniline
c) p-bromoaniline
d) 2, 4, 6-tribromoaniline
View Answer
Explanation: Aniline on treatment with excess of bromine water is a nucleophilic substitution reaction and will form 2, 4, 6-tribromoaniline.
14. When an organic compound was treated with sodium nitrite and hydrochloric acid in the ice cold, nitrogen gas was evolved copiously. What is the compound?
a) A nitrocompound
b) A primary amine
c) An aliphatic primary amine
d) An aromatic primary amine
View Answer
Explanation: When an aliphatic primary amine was treated with sodium nitrite and hydrochloric acid in the ice cold, nitrogen gas was evolved.
15. Primary amines react with nitrous acid to yield which of the following?
a) Insoluble nitrite salts
b) Yellow oily layer
c) Nitrogen gas
d) Azo dye
View Answer
Explanation: Primary amines react with nitrous acid to yield Nitrogen.
CH3 − NH2 + HNO2 → CH3OH + N2 + H2O
16. The reaction of HNO2 with ‘A’ gives quaternary ammonium salt. A is which of the following?
a) Methyl amine
b) Dimethyl amine
c) Trimethyl amine
d) Aniline
View Answer
Explanation: The reaction of HNO2 with trimethyl amine gives quaternary ammonium salt.
R3N + HONO → R3N . HONO
Sanfoundry Global Education & Learning Series – Organic Chemistry.
To practice all areas of Organic Chemistry, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
- Check Organic Chemistry Books
- Check Chemical Engineering Books