This set of Class 11 Physics Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Thermodynamics”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. ΔU=0 means that the process is isothermic. True or False?
a) True
b) False
View Answer
Explanation: Internal energy is a function of temperature only. So if ΔU=0 implies that the process will have constant temperature, or it is isothermic.
2. First law of thermodynamics is based on?
a) Conservation of energy
b) Conservation of mass
c) Conservation of momentum
d) Conservation of work
View Answer
Explanation: The first law of thermodynamics is based on the conservation of energy. It deals with work done and heat energy supplied or removed from a system. It basically says that energy supplied to a system is conserved.
3. If 315cal of heat is given to the system, and the system does 20cal of work, find the change in internal energy.
a) 295cal
b) 335cal
c) 0 cal
d) 335J
View Answer
Explanation: The first law states that ΔU=ΔQ – ΔW
= 315 – 20
= 295cal.
Work is positive as it is done by the system. Heat is positive as it is supplied to the system.
4. What is the relation between the internal energy and heat supplied in the process 1 & 2 shown in the diagram? Both paths start at A and end at B.
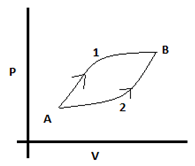
a) U1 > U2, Q1 > Q2
b) U1 < U2, Q1 > Q2
c) U1 = U2, Q1 = Q2
d) U1 = U2, Q2 > Q2
View Answer
Explanation: The initial and final states are the same for both processes, so the value of internal energy will be the same (U1 = U2).
The area under the PV curve gives work done.
In the given diagram, area under 1 is greater than area under 2. So, W1 > W2.
We know, ΔQ =ΔU + ΔW, which implies that Q1 > Q2 since U is same for both and W1 > W2.
5. A block of mass 1kg is given a velocity of 5m/s on a horizontal flat surface. What will be the change in internal energy of the block-surface system, when the block comes to rest. The coefficient of kinetic friction is 0.3 and coefficient of static friction is 0.4.
a) 25J
b) 12.5J
c) 0
d) 30J
View Answer
Explanation: The kinetic energy of the block will change into internal energy of the system.
Initial kinetic energy = 0.5*1*25 = 12.5J.
Therefore the internal energy change = 12.5J.
6. Consider a gas contained in a rigid container of volume 20m3. What will be the change in internal energy if 50J of heat is provided to it? Assume the gas exerts a pressure of 1atm on the walls.
a) 50J
b) 0
c) 70J
d) 20J
View Answer
Explanation: All the heat supplied will be equal to change in internal energy.
The work done is zero because the container is rigid hence no volume change.
Thus, ΔU = ΔQ – ΔW = 50 + 0 = 50J.
7. A disc spinning with 24J of kinetic energy is kept in a container containing a fluid having a heat capacity of 2000J/K. When the disc comes to rest what will be the change in internal energy if ΔU = 3.5ΔT.
a) 0
b) 0.042J
c) 0.021J
d) 0.028J
View Answer
Explanation: When the disc comes to rest all its kinetic energy will have been converted into internal energy of the system.
The fluid has a heat capacity of 2000J/K which means that for 24J the change in temperature will be 24/2000 = 0.012K.
Given: ΔU = 3.5ΔT
⇒ ΔU = 3.5*0.012
= 0.042J.
8. In the process A to B to C, 20J of heat is supplied from A to B. 20.5J of heat has been removed from B to C and 2J of heat has been added from C to A. Calculate the value of ‘v’ from the given conditions in the diagram. The values of pressure and volume given in the graph are in S.I. units.
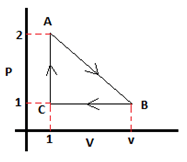
a) 4m3
b) 1.5m3
c) 3m3
d) 8m3
View Answer
Explanation: In one cycle the change in internal energy should be zero.
The heat energy supplied in one cycle is 20 – 20.5 + 2 = 1.5J.
Therefore the work done by the system in one cycle should be 1.5J.
Area under PV curve is work done,
thus 0.5*(v-1)*1 = 1.5.
∴ v = 4m3.
9. In the given system, 20J of heat is supplied to the gas molecules. The spring is initially not elongated or compressed. If the spring gets compressed by 1cm. Calculate the change in internal energy of the system. Spring constant = 200N/m. Assume piston moves slowly.
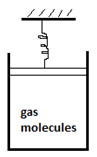
a) 19J
b) -19J
c) 21J
d) -21J
View Answer
Explanation: The work done by the piston will be stored in spring.
The energy stored in spring = 1/2kx2 = 0.5*200*0.01 = 1J.
The work done by piston = 0.01J.
ΔU = ΔQ – ΔW = 20 – 1 = 19J.
10. At constant pressure P the volume of a gas increases from V1 to V2 when ‘Q’ amount of heat is removed from the system. What will happen to internal energy?
a) Remain the same if Q = P(V1 – V2)
b) Decrease by an amount P(V2 – V1) – Q
c) Decrease by an amount Q + P(V2 – V1)
d) Increase by some unknown amount
View Answer
Explanation: The internal energy will definitely decrease as both factors heat removal and work done by gas support the same. ΔU = ΔQ – ΔW = -Q – P(V2 – V1) = -(Q + P(V2 – V1)). Thus, we can say that internal energy decreases by an amount = Q + P(V2 – V1).
More MCQs on Class 11 Physics Chapter 12:
- Chapter 12 – Thermodynamics MCQ (Set 2)
- Chapter 12 – Thermodynamics MCQ (Set 3)
- Chapter 12 – Thermodynamics MCQ (Set 4)
- Chapter 12 – Thermodynamics MCQ (Set 5)
- Chapter 12 – Thermodynamics MCQ (Set 6)
- Chapter 12 – Thermodynamics MCQ (Set 7)
- Chapter 12 – Thermodynamics MCQ (Set 8)
- Chapter 12 – Thermodynamics MCQ (Set 9)
- Chapter 12 – Thermodynamics MCQ (Set 10)
- Chapter 12 – Thermodynamics MCQ (Set 11)
To practice all chapters and topics of class 11 Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Mathematics MCQs
- Check Class 11 - Physics Books
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Chemistry MCQs
- Practice Class 11 - Biology MCQs
