This set of Class 11 Physics Chapter 13 Multiple Choice Questions & Answers (MCQs) focuses on “Kinetic Theory”. These MCQs are created based on the latest CBSE syllabus and the NCERT curriculum, offering valuable assistance for exam preparation.
1. What is the number of molecules in 2.24L of SO2 at STP?
a) 6.023*1023
b) 6.023*1022
c) 6.023*1020
d) 6.023*1021
View Answer
Explanation: According to Avogadro’s law 22.4L of any gas at STP is 6.023*1023.
So, in 2.24L there will be 6.023*1023/10
= 6.023*1022.
2. What is the mass of 22.4L of CO2at STP?
a) 1g
b) 44g
c) 44kg
d) 1kg
View Answer
Explanation: 22.4L of a gas at STP has a weight equal to its molar mass.
So, the weight of CO2 will be 12+16+16
= 44g.
3. The behavior of real gases approaches that of ideal gas in which of these following conditions?
a) Low pressure & low temperature
b) High Pressure & high temperature
c) Low pressure & high temperature
d) high pressure & low temperature
View Answer
Explanation: Ideal gas is based on the assumptions of kinetic theory of gases(KTG). At low pressure and high temperature the intermolecular forces become less significant and the size of molecules becomes less as compared to separation between them. These are two postulates of KTG and hence in these conditions real gas behavior is similar to that of ideal gases.
4. There are 3 non-interacting ideal gases in a container. The moles of gases 1,2 & 3 are in the ratio 1:3:5. If the total pressure is 54Pa, find the value of partial pressure of gas 1.
a) 6Pa
b) 12Pa
c) 18Pa
d) 28Pa
View Answer
Explanation: The ratio of partial pressures will be in the same ratio as that of moles, i.e: 1:3:5.
Let the partial pressure of gas 1 be ‘x’.
Thus, x+3x+5x = 54.
x = 6Pa.
5. What is the ratio of densities of 2 gases, O2 & N2, having partial pressures in the ratio 2:3?
a) 16/21
b) 12/7
c) 21/16
d) 7/12
View Answer
Explanation: The ratio of moles is the same as the ratio of partial pressures.
The ratio of densities:
d1/d2= (m1/V)/(m2/V)
= m1/m2= (n1M1)/(n1M2),
where n is the number of moles and M is the molecular mass. d1/d2= (2/3)*(16/14)
= 16/21.
6. In the given diagram, one graph is of an ideal gas and another is of a real gas. Select the correct option.
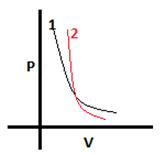
a) 1-real gas, 2-ideal gas
b) 1-ideal gas, 2-real gas
c) Both are for an ideal gas at different temperatures
d) Their graphs cannot intersect
View Answer
Explanation: Real gases, unlike ideal gases, consider volume taken up by molecules because of which mean free path decreases or collisions increase and hence pressure increases. So, at low volumes this factor will play a big role and thus for a particular volume in the low volume range, pressure of real gases will be higher. To see which graph belongs to whom we can draw a line parallel to the pressure axis at very low volume. The one having higher pressure will be that of real gases. Therefore, 2-real gas & 1-ideal gas.
More MCQs on Class 11 Physics Chapter 13:
- Chapter 13 – Kinetic Theory MCQ (Set 2)
- Chapter 13 – Kinetic Theory MCQ (Set 3)
- Chapter 13 – Kinetic Theory MCQ (Set 4)
To practice all chapters and topics of class 11 Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 11 - Biology MCQs
- Check Class 11 - Physics Books
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Mathematics MCQs
