This set of Class 11 Physics Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Thermodynamic Processes”.
1. In which of the following processes is heat transfer equal to zero?
a) Isentropic
b) Isochoric
c) Isothermal
d) Diathermic
View Answer
Explanation: Entropy is defined as dQ/T. In an isentropic process dQ/T = 0 which means dQ = 0. Diathermic is a process in which heat flow is easily possible. Isochoric process is one in which volume is constant. Isothermal process is one in which temperature stays constant.
2. Isothermal process can be represented by which law?
a) Charle’s law
b) Boyle’s law
c) Gay-Lussac’s law
d) 2nd law of thermodynamics
View Answer
Explanation: In an isothermal process, PV=const. This is the same as Boyle’s law. Charle’s law is given by: V/T=const. Gay-Lussac’s law is given by: P/T=const and 2nd law of thermodynamics states that in every process total entropy of the universe must increase.
3. In an isothermal process for an ideal gas, change in internal energy is zero. True or False?
a) True
b) False
View Answer
Explanation: For an ideal gas internal energy depends only on temperature. In an isothermal process change in temperature is zero, hence internal energy also remains the same.
4. Calculate the work done by the gas in an isothermal process from A to B. PA = 1Pa, VA = 3m3, PB = 3Pa.
a) 3.3J
b) 3J
c) -3.3J
d) -4.58J
View Answer
Explanation: Since the process is isothermal the product PV will be constant.
PAVA = PBVB.
∴ VB = 1*3/3 = 1m3.
Work done in an isothermal process is given by:
nRT*ln(VB/VA)
= PAVAln(VB/VA)
= 3ln(1/3)
= -3.3J.
5. Find the value ofVC. PA = 2atm, PB = 6atm, PC = 12atm, VA = 1L. The process AB and CD is isothermal while BC and DA is adiabatic. For adiabatic processes, take γ =1.3.
a) 0.196L
b) 0.243L
c) 0.296L
d) 0.187L
View Answer
Explanation: From A to B we can use PAVA = PBVB since the process is isothermal.
∴ VB = 2/6 = 1/3L.
From B to C we use PV1.3 = constant since it is an adiabatic process. PBVA
1.3 = PCVC1.3.
∴ VC1.3 = (6*0.24)/12 = 0.12.
∴ VC = 0.196L.
6. Which of the following variables is zero for a cyclic process?
a) Work done
b) Heat supplied
c) Total heat + Total work
d) Total heat – Total work
View Answer
Explanation: In a cyclic process, the starting position is the same as the ending position. So, the change in all state variables is zero. So the net change in internal energy is zero. ΔU = ΔQ – ΔW = 0.
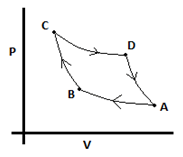
7. In the given diagram, one process is adiabatic and the other is isothermal. It can be said that process AB is adiabatic. True or False?
a) True
b) False
View Answer
Explanation: The slope of adiabatic curve in a PV diagram is γ times that os isothermal curve. And γ is always greater than one, so the slope of adiabatic is greater than isothermal. Therefore, AB has to be isothermal and AC has to be adiabatic.
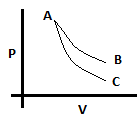
8. When the state changes from A to B, what will happen to internal energy?
a) Increase
b) Decrease
c) Remain same
d) Depends on value of heat supplied
View Answer
Explanation: On going from A to B the temperature will increase because the isotherm passing through B will be further away from the origin than the given isotherm passing through A and will therefore be at a higher temperature. As the temperature increases we can say that internal energy will also increase.
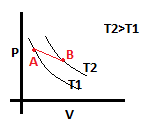
9. What is the amount of heat supplied in one cycle?
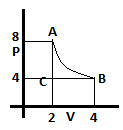
a) 3.2J
b) -3.2
c) 4.8J
d) -4.8J
View Answer
Explanation: In one cycle the work done will be equal to the heat supplied, since change in internal energy is zero in a cyclic process.
Work done = nRT*ln(VB/VA) – PB (VB – VA)
= PAVA*ln(VB/VA) – PB (Vb – VA)
= 16*ln(4/2) – 4(4-2) = 16*0.7 – 8 = 3.2J.
Thus, heat supplied = 3.2J.
10. What is the relation between heat supplied in the 3 processes?
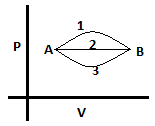
a) Q1 > Q2 > Q3
b) Q1 < Q2 < Q3
c) Q1 = Q2 = Q3
d) Q1 = Q3 > Q2
View Answer
Explanation: The internal energy is the same for all 3 paths. And Δ U = Δ Q – Δ W, so more the value of Δ W, more will be the value of Δ Q. Path 1 has the maximum area under its curve, followed by path 2 and then path 3. Work done is area under PV curve, hence: W1 > W2 > W3. So, the relation for heat will also be: Q1 > Q2 > Q3 since Q – W should be the same for all 3 processes.
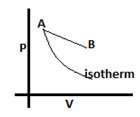
11. The given graph corresponds to which equation?
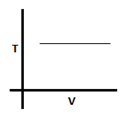
a) V = 0
b) PV = constant
c) V/T = constant
d) PT = constant
View Answer
Explanation: In the given graph temperature remains constant with variation in volume. So the process is isothermal and PV=constant.
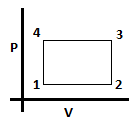
12. What is the expression for work done in the given cycle? Let the number of moles of gas be ‘n’. Process starts at 1 and ends at 4 going in the clockwise direction.
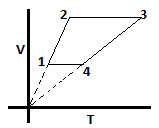
a) 0
b) nR(T4/V1 – T1/V1) (V1 -V2)
c) nR(T4/V4 – T1/V1) (V2 – V1)
d) nR(T4/V3 – T1/V2) (V1 – V2)
View Answer
Explanation: First, we should draw the PV diagram. From the PV diagram we see that work done will be negative as positive work is done under path 1 to 2 and negative work under path 3 to 4. Expression for work will be: (P4 – P1)(V1 – V2) = nR(T4/V4 – T1/V1) (V1 – V2). Also V4 = V1.
Sanfoundry Global Education & Learning Series – Physics – Class 11.
To practice all chapters and topics of class 11 Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 11 - Mathematics MCQs
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Biology MCQs
- Check Class 11 - Physics Books
