This set of Class 11 Physics Chapter 12 Multiple Choice Questions & Answers (MCQs) focuses on “Thermodynamics – Carnot Engine”.
1. Find work done by one mole of a gas in one cycle. V1 = 1m3, V2 = 4m3, V3 = 6m3, V4 =3m3. Temperature is given in °C.
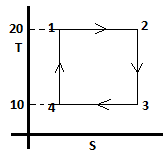
a) 303Rln(4) J
b) 303Rln(2) J
c) 30Rln(2) J
d) 30Rln(4) J
View Answer
Explanation: The given T-S diagram represents a carnot cycle.
Work done is given by:
nRT1ln(V2/ V1) – nRT4ln(V3/ V4)
= 293Rln(4) – 283Rln(2)
= 303Rln(2).
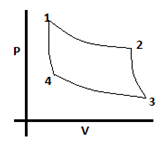
2. What is the correct order of steps for a carnot cycle?
a) Isothermal expansion, adiabatic expansion, isothermal compression, adiabatic compression
b) Isothermal compression, adiabatic expansion, isothermal expansion, adiabatic compression
c) Adiabatic compression, adiabatic expansion, isothermal expansion, isothermal compression
d) Adiabatic compression, isothermal expansion, adiabatic expansion, isothermal compression
View Answer
Explanation: A carnot cycle is a reversible cyclic process having the maximum possible efficiency. The steps of the cycle are: isothermal expansion of gas, adiabatic expansion, isothermal compression and then adiabatic compression. This is evident from its PV diagram:
3. What will be the value of temperature of hot reservoir in a carnot cycle, if the temperature of cold reservoir is 25°C, heat absorbed from hot reservoir is 12J and heat rejected to cold reservoir is 4J?
a) 684K
b) 333.33K
c) 894K
d) 433.33K
View Answer
Explanation: For a carnot cycle: Q2/Q2 = T2/T1.
T2 = 12/4 * 298 = 894K.
4. A carnot engine extracts 1200J of energy from a hot reservoir. What is the amount of work done by it? Temperatures of hot and cold reservoirs are 200K and 150K respectively.
a) 800J
b) 900J
c) 600J
d) 850J
View Answer
Explanation: The efficiency of a carnot engine is:
1- T2/T1 = 1-150/200 = 1/4.
Efficiency is also given by: 1 – Q2/Q1 = 1 – Q2/1200 = 1/4.
Q2 = 3/4 *1200 = 900J.
5. The efficiency of a carnot engine is 50%. The temperature of the hot reservoir is kept constant. By what amount should the temperature of the cold reservoir be decreased so that efficiency becomes 60%.
a) 1K
b) 2K
c) 30K
d) 83K
View Answer
Explanation: 0.5 = 1 – TC/TH.
And 0.6 = 1 – (TC + x)/TH.
From the first equation we get: 5/10 = TC/TH.
From the second equation we get: 4/10 = (TC + x)/TH.
Now, we see that the value of x must be 1K.
Sanfoundry Global Education & Learning Series – Physics – Class 11.
To practice all chapters and topics of class 11 Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 12 - Physics MCQs
- Practice Class 11 - Mathematics MCQs
- Check Class 11 - Physics Books
- Practice Class 11 - Biology MCQs
- Practice Class 11 - Chemistry MCQs
