This set of Class 11 Physics Chapter 11 Multiple Choice Questions & Answers (MCQs) focuses on “Thermal Properties of Matter – Specific Heat Capacity”.
1. If we supply equal amounts of heat to equal masses of two different substances, the rise in temperature will be the same for both. True or False?
a) True
b) False
View Answer
Explanation: Each body has a heat capacity which indicates the amount of heat it requires to raise its temperature by 1°C. Different substances require different amounts of heat for the same rise in temperature.
2. Why is water used in automobiles as a coolant?
a) It is not toxic for the environment
b) It has a high specific heat capacity
c) It has a high lubricating property which in turn keeps the engine cool by reducing friction
d) It is available in abundance
View Answer
Explanation: Water has a high specific heat capacity because of which it can absorb large amounts of heat before increasing its temperature. Hence, it is used as a coolant.
3. Specific heat capacity depends on the mass of the substance. True or False?
a) True
b) False
View Answer
Explanation: Specific heat capacity is defined as the heat capacity per unit mass for a substance. s = ΔQ/mΔT. So, no matter what mass of a substance we take ‘s’ will be equal to (ΔQ/ΔT) divided by that mass, which means ‘s’ doesn’t depend on the mass.
4. CP > CV always. True or False?
a) True
b) False
View Answer
Explanation: When heat is added at constant pressure the heat is used to increase temperature and increase volume or we can say does work. But when heat is added at constant volume the heat only increases temperature and volume remains the same (work = 0). So For the same rise in temperature at constant pressure we have to provide more heat.
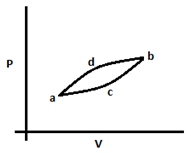
5. What is the relation between molar heat capacities of the two processes given in the diagram below? Assume process acb has a heat capacity of C1 and process adb has a heat capacity of C2.
a) C1 > C2
b) C2 > C1
c) C1 = C2
d) C1 = C1/ Area inside curve
View Answer
Explanation: As the start and end points are the same for both processes, the temperature rise will be the same. The work done in process adb is more than that done in process acb. Therefore heat supplied will be less in process acb, so C2 > C1.
6. A closed container contains 0.6m3 of neon gas at 200K temperature and 1.3*105Pa. Find the rise in temperature when 8360 J of heat is supplied to it. The molar heat capacity of neon at constant volume is 3.2calK-1mol-1. Assume the container doesn’t expand on heating.
a) 13.3K
b) 15.8K
c) 13.9K
d) 15K
View Answer
Explanation: no. of moles of neon gas = pV/RT
= (1.3*105*0.6) / (8.31*200)
= 46.9 moles ≅ 47 moles
ΔQ = nCVΔT ΔT = ΔQ/nCV
= (8360/4.18) / (47*3.2)
= 13.3K.
7. CV of a gas is 8 calK-1mol-1. Find CP/CV. Assume R = 2 calK-1mol-1.
a) 1.4
b) 1.33
c) 1.25
d) 1.8
View Answer
Explanation: CP = CV + R = 8 + 2
= 10 calK-1mol-1.
CP/CV= 10 / 8 = 1.25.
Sanfoundry Global Education & Learning Series – Physics – Class 11.
To practice all chapters and topics of class 11 Physics, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Class 11 - Chemistry MCQs
- Practice Class 11 - Mathematics MCQs
- Check Class 11 - Physics Books
- Practice Class 11 - Biology MCQs
- Practice Class 12 - Physics MCQs
