This set of Bioseparation Processes Multiple Choice Questions & Answers (MCQs) focuses on “Precipitation Factors”.
1. At which pH the minimum solubility can be observed?
a) Isoelectric point
b) pH 2
c) pH 7
d) pH 9
View Answer
Explanation: The solubility of protein depends on the pH of the solution and the minimum solubility is observed when the pH of solution becomes neutral and no more precipitation is possible for the solutes i.e. at isoelectric point.
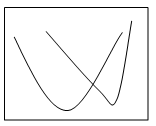
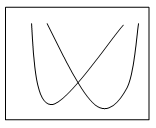
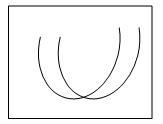
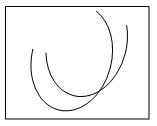
2. Which of the following graphs shows the pH-solubility curves for two proteins A and B?
a)
b)
c)
d)
View Answer
Explanation: The solubility of a protein at Isoelectric point is the minimum and the graph b shows the pH solubility curves between two proteins and the separation of protein is carried out through maintaining the pH of the solution at Isoelectric point of one protein. At this condition, the protein at Isoelectric point would have been precipitated more than the one still remaining in the solution.
3. Which process of precipitation can utilize the pH-solubility effect?
a) Solvent based process
b) Base based process
c) Salt based process
d) Acid based process
View Answer
Explanation: The pH-solubility effect can be utilized in the salt based protein precipitation process for the optimization of operating conditions and pH adjustment in cold environment is rarely used in the process of protein separation therefore, the solubility difference is not much between the proteins in a solution.
4. Which parameter is involved in precipitation of protein by using ethanol and acetone?
a) pH of the solution
b) Temperature of the solution
c) Solubility of the solutes
d) Dielectric constant of the solution
View Answer
Explanation: The use of ethanol in the process of protein precipitation leads to decrease in the dielectric constant in which the salt and ethanol are added in the aqueous solution and it leads to de-salting of the solution and finally the protein precipitates.
5. What is the cause of protein purification based on the solubility as well as the size of the molecules in the solvent?
a) Addition of non-ionic polymer
b) Addition of ionic polymer
c) Addition of solvents
d) Addition of acid
View Answer
Explanation: The size of protein purification with respect to solubility is caused by addition of non-ionic polymer; it results in steric exclusion of protein molecule from the volume of solution occupied by the polymer.
6. Who developed the model for protein purification based on solubility of the protein?
a) Fick
b) Juckes
c) Kick
d) Raman
View Answer
Explanation: Juckes developed the model for this phenomenon based on the protein molecule being in a form of a solid sphere as well as the polymer molecule in the form or a rod shape.
7. What is the equation of solubility of the protein based on Juckes model?
a) -ln S = β’ – K’ cp
b) ln S = -β’ – K’ cp
c) ln S = β’ – K’ cp
d) ln S = β’ + K’ cp
View Answer
Explanation: The equation for solubility of protein is ln S = β’ – K’ cp where, K’ = \(\frac{V}{2.303} (\frac{r_s + r_s}{r_s})^3\), rs and rr are the radius of the protein solute and polymer rod respectively, V is the partial specific volume of the polymer, cp is the polymer concentration and β’ is a constant and it is observed that this model results in lowest protein solubility for large proteins.
8. Which type of precipitation has molecular weight as a predominant factor?
a) Column precipitation
b) Polymer precipitation
c) Solute precipitation
d) Affinity precipitation
View Answer
Explanation: The affinity precipitation is based on the molecular size of the solute. When the affinity groups or antibodies to a specific biomolecules are added to a solution, the antigen-antibody interaction can form large multi-molecular complexes. These complexes are insoluble and cause selective precipitation of the antigen. The average size of the complex agglomerates is increased when there is a stoichiometric ratio of antibody and antigen.
9. Which equation is used to describe the salting out effect of the protein?
a) Cohn’s equation
b) Flick’s equation
c) Kick’s equation
d) Juckes equation
View Answer
Explanation: The salting out effect of the protein is explained by Cohn’s equation which is followed at high ionic strength of the protein. The dielectric constant decreases with decrease in polarity of the solvent. The salting out effect tends to predominate in the non-polar solvents while the salting out effect is more dominant in aqueous solvents.
10. What is cohn’s equation?
a) ln S = β’ + K’ I
b) ln S = β – K’ I
c) – ln S = β’ – K’ I
d) -ln S = β’ – K’ c
View Answer
Explanation: The cohn’s equation is ln S = β – K’ I where, S is the solubility of the protein, β is the constant which has a hypothetical solubility of the protein at zero ionic strength, K’ is the salting out constant characteristics of the specific protein and the salt which is independent of temperature and pH above the equilibrium point i.e. Isoelectric point.
11. What is Kirkwood equation?
a) ln Sp = ln S0 – (Ks– Ki)I
b) ln Sp = ln S0 + (Ks – Ki)I
c) ln S = β – K’ I
d) ln S = β’ – K’ cp
View Answer
Explanation: Kirkwood equation is the rearranged equation obtained from Cohn’s equation and Juckes equation. It is used for estimation of solubility of dipolar ions. It can be used to obtain the identical form of Cohn equation like β = ln S0.
12. When ammonium sulphate was added to protein the protein precipitated with the initial concentration of 15g/l protein and the concentration of ammonium sulphate was 0.5 M and 1.0M. Estimate the concentration of ammonium sulphate when the concentration of protein in the fermentation broth was 13.5g/l and 15.0g/l respectively. Note: The recovery of protein was 95%.
a) 2.5M
b) 3.5 M
c) 1.95 M
d) 5M
View Answer
Explanation: Using Cohn’s equation, ln S = β – K’ I the experimental data into this equation gives ln 13.5 = β – 0.5 K’ and ln 5 = β – 1.5K’ so the value of β = 3.6 and K’ = 1.99 M-1. For 95% recovery, the concentration will be c = \(\frac{\beta – ln S}{K’}\) = \(\frac{3.6 – ln(0.05 × 15)}{1.99}\) = 1.95M.
Sanfoundry Global Education & Learning Series – Bioseparation Processes.
To practice all areas of Bioseparation Processes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
