This set of Bioseparation Science Multiple Choice Questions & Answers (MCQs) focuses on “Chromatography Theory – Set 2”.
1. What are the two components of retention time in the process of chromatography?
a) tM
b) tR’
c) tR
d) tM and tR’
View Answer
Explanation: The two components of retention time is the mobile phase retention time i.e. tM, it is within a column which is based purely on hydrodynamic considerations, and the another component is the adjusted retention time tR‘, it is due to solute-stationary phase interaction tR = tM + tR‘.
2. The mobile phase retention time depends on _____________
a) flow rate
b) column volume
c) voidage fraction, flow rate, column volume
d) voidage fraction
View Answer
Explanation: The mobile phase retention time is dependent on the flow rate of mobile phase, the volume of column and the voidage fraction. tM = \(\frac{V_c \varepsilon}{Q}\) Where, Q is the flow rate of mobile phase, tM is the retention time of mobile phase and Vc is the volume of column.
3. The solute concentration in a pulse is ____________
a) rectangular
b) pentagonal
c) hexagonal
d) spherical
View Answer
Explanation: The concentration of solute in a pulse is rectangular in shape and it means that the presence of the concentration has a constant value and rest at other places it is zero. Once the solute crosses the column, the solutes appears in the effluent and hence in the chromatograms in form of a peak.
4. The transformation in the shape of chromatograms is due to ___________
a) interaction
b) distribution, interaction
c) dispersion, interaction, distribution
d) dispersion
View Answer
Explanation: The transformation in the shape of chromatograms is due to interaction of solute with the stationary phase, a non-ideal distribution of the inlet, radial dispersion as well as axial dispersion and Golay-Taylor dispersion.
5. What is the significance of the given diagram?
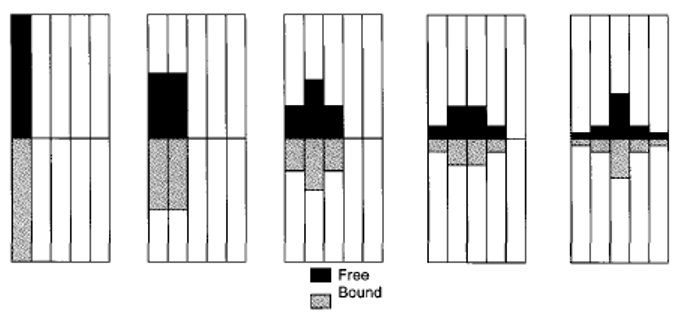
a) Partitioning of a solute
b) Partitioning of solvent
c) Column
d) Mobile phase
View Answer
Explanation: The given image is of partitioning of a solute within column. The darker shade in the image represents the moles of solute in the mobile phase and the lighter shade represents the moles of solutes that are bound to solute. The solute distribution corresponding to five time increments is shown. As the pulse of the solute moves through the column, its shape changes to that of a peak.
6. The change in shape of the peaks is due to ___________
a) stationary phase
b) interaction of solute
c) mobile phase
d) interactions with mobile phase
View Answer
Explanation: The change in shape of the peaks is due to solute interaction with the stationary phase. The interaction marks in the dynamic circulation of the solute between the mobile as well as stationary phase during the movement of solute through the column.
7. What is the significance of the given diagram?
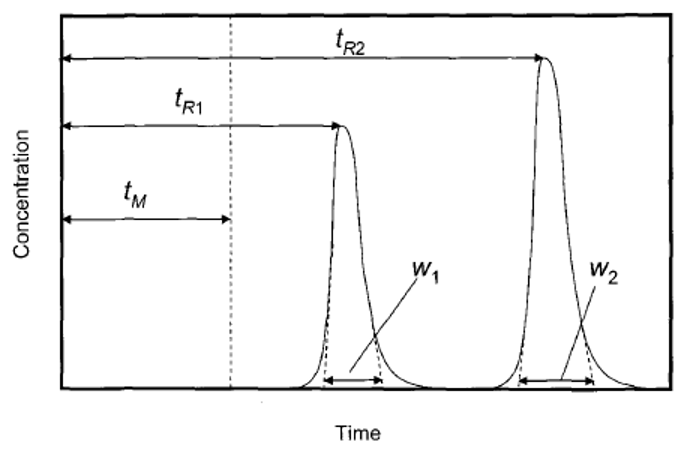
a) Peak separation in chromatogram
b) Mobile phase
c) Solute display
d) Stationary phase
View Answer
Explanation: A peak of chromatogram has been shown in the image, chromatographic column has been visualized as consisting of five segments, within each of which the solutes can distribute between the mobile and stationary phases.
8. The spatial separation between two peaks is measured in terms of ___________
a) column properties
b) mobile phase flow rate
c) resolution parameter
d) retention time
View Answer
Explanation: The spatial separation to the two peaks means whether the peaks overlap each other or not, is measured in terms of the resolution parameter R = \(\frac{t_{R2} – t_{R1}}{0.5(w_1 + w_2)}\) where tR1 is retention time of solute eluted first and tR2 is retention time of solute eluted second, w1 is peak width of solute eluted first and w2 is peak width of solute eluted second.
9. The number of theoretical plates in a chromatographic column can be determined by ___________
a) chromatogram
b) solute flow rate
c) mobile phase flow rate
d) column type
View Answer
Explanation: The number of theoretical plates in a chromatographic column can be unwavering by obtaining a chromatogram with a solute. N is the number of plates and it depends on the retention time of solute and width peak. N = 16(\(\frac{t_R}{w})^2\).
10. The height of theoretical plate can be determined by ___________
a) Diffusion
b) Deemter equation
c) Transfer coefficient
d) Velocity of mobile phase
View Answer
Explanation: Height of theoretical plate is determined by using Deemter equation and it is H = A + \(\frac{B}{u}\) + Lu where A is eddy diffusivity component, B is the axial diffusion constant, L is the transfer constant and u is the velocity of mobile phase.
11. Calculate the mobile phase retention time for a plasmid having retention time of 10min and the volume of 0.01 m3 having voidage fraction of 0.3. (given: K is 2)
a) 1.76 min
b) 1.86 min
c) 1.96 min
d) 2 min
View Answer
Explanation: tR = tM (1 + \(\frac{1 – \varepsilon}{\varepsilon}\) K) so, 10 = tM (1 + \(\frac{1 – 0.3}{0.3}\) × 2) ∴ tM is 1.76 min.
12. Calculate the number of theoretical plates in the column for albumin retention time of mobile phase 0.975min, retention time is 1.27 min having peak of albumin at 0.52.
a) 90
b) 92
c) 94
d) 95
View Answer
Explanation: N = 16(\(\frac{t_R}{w})^2\) so, number of theoretical plates N = 16(\(\frac{1.27}{0.52})^2\) = 95.
Sanfoundry Global Education & Learning Series – Bioseparation Science.
To practice all areas of Bioseparation Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
