This set of Bioseparation Processes Multiple Choice Questions & Answers (MCQs) focuses on “Staged Counter-Current Extraction”.
1. Which extraction technique can be used as a replacement of the extraction process which causes the diminishing of concentration?
a) Staged counter-current extraction
b) Cross-current extraction
c) Batch extraction
d) Continuous extraction
View Answer
Explanation: The staged counter-current extraction process is used as substitute for the cross-current extraction, it is referred as form of chain of extraction through current cascades.
2. How the feed stream does enters the nth stage of the staged counter current extraction?
a) Extracting solvent enters 0th stage
b) Extracting solvent enters 2nd stage
c) Extracting solvent enters nth stage
d) Extracting solvent enters 1st stage
View Answer
Explanation: The Raffinate phase is collected from the 1st stage while the extract phase is collected from the nth stage. Using the method of staged counter current process of extraction, the concentration driving force is maintained more or less uniform in all the stages of extraction comprising the cascade.
3. What are the basic assumptions required for obtaining analytical solution for the process of extraction based on the staged counter current?
a) Equilibrium at all stages, Negligible entrainment of other phases, Same equilibrium for all stages
b) Same equilibrium for all stages, Negligible entrainment of other phases, K is independent of concentration of solute
c) K is independent of concentration of solute, Same equilibrium for all stages, Equilibrium at all stages
d) Negligible entrainment of other phases, Same equilibrium for all stages, K is independent of concentration of solute
View Answer
Explanation: The basic assumptions involved in the obtaining of analytical solution for the process of extraction based on the staged counter current are the Raffinate as well as extract streams from each stage are in equilibrium, there is negligible entrainment of the other phases and the same equilibrium relationship is sufficient for all the stages of extraction process which means that the value of K is independent of concentration of solute.
4. What is the significance of the given diagram?
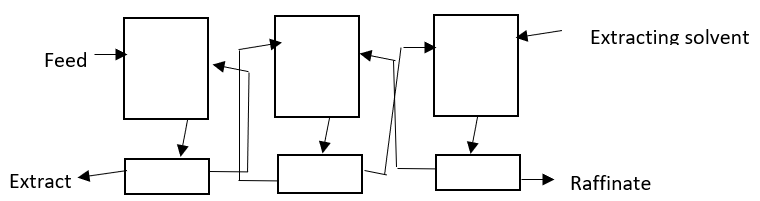
a) Staged non-counter current extraction
b) Staged current extraction
c) Staged counter current extraction
d) Batch current extraction
View Answer
Explanation: The diagram is to explain the staged counter current process of extraction in which the units are expressed at three different stages of the extraction, starting from the feed material entering in the first unit and continuing in the various stages and finally the extract is separated out.
5. What is the solute material balance equation for the first stage of the extraction process?
a) QRCR2 = QRCR1 + QECE1
b) QRCR = QRCR1 + QECE1
c) QRCR1 = QRCR1 + QECE1
d) QRCR2 = QRCR2 + QECE1
View Answer
Explanation: The flow of the feed at final stage is equal to the flow of feed at initial stage along with the concentration of raffinate added to the flow of raffinate along with the concentration of the extract at first stage.
6. How will you calculate the concentration of the raffinate at second stage of the process of extraction?
a) CR2 = -(λ + 1)CR1
b) CR2 = (λ – 1)CR1
c) CR2 = (-λ + 1)CR1
d) CR2 = (λ + 1)CR1
View Answer
Explanation: The concentration of the raffinate at second stage can be calculated by using the feed flow along with the concentration of the raffinate at first stage, so that the continuous process can be maintained depending on the concentration of feed at the beginning of the extraction process and by the end of the process the concentration of the raffinate.
7. What will be the extraction factor of the process of extraction for the first stage of the extraction under the staged counter current process of extraction?
a) λ = –\(\frac{K Q_E}{Q_R}\)
b) λ = \(\frac{K Q_E}{Q_R}\)
c) -λ = \(\frac{K Q_E}{Q_R}\)
d) λ = \(\frac{K – Q_E}{Q_R}\)
View Answer
Explanation: The extraction factor for the process of extraction for the first stage of the extraction under the staged counter current process of extraction is equal to the product of equilibrium constant and the flow rate of the extract divided by the flow rate of the raffinate at the first stage of the extraction.
8. What will be the equation for the solute material balance over the second stage of the staged counter current process of extraction?
a) CR3 = (1 – λ + λ2)CR1
b) CR3 = (1 + λ – λ2)CR1
c) CR3 = (λ + λ2)CR1
d) CR3 = (1 + λ + λ2)CR1
View Answer
Explanation: The concentration of the raffinate at final stage of the extraction process is equal to the sum of extraction factor and the square of extraction factor along with the product of the concentration of the raffinate at initial stage of the staged counter current process of extraction.
9. What will be the concentration of the raffinate at the nth stage of the extraction process in the staged counter current of extraction?
a) CRn+1 = \((\frac{\lambda^{n+1}-1}{λ-1})\) – CR1
b) CRn+1 = \((\frac{\lambda^{n+1}-1}{λ-1})\) CR1
c) CRn+1 = \((\frac{\lambda^{n+1}-1}{λ+1})\) CR1
d) CRn+1 = \((\frac{\lambda^{n+1}-1}{λ-1})\) + CR1
View Answer
Explanation: The concentration of the raffinate at the nth stage of the extraction process in the staged counter current of extraction is the product of the concentration of the raffinate at the first stage of the extraction process along with the divided amount of the extraction factor at nth time to the 1 extraction factor less than the original value obtained.
10. How to estimate the fraction extracted by the process of staged counter current process of extraction?
a) p = \(\frac{Q_E C_{En}}{Q_R C_{Rn+1}}\)
b) p = \(\frac{Q_E – C_{En}}{Q_R C_{Rn+1}}\)
c) p = \(\frac{Q_E C_{En}}{Q_R – C_{Rn+1}}\)
d) p = \(\frac{Q_E – C_{En}}{Q_R – C_{Rn+1}}\)
View Answer
Explanation: The final product obtained at the end of the staged counter current extraction process is the product of flow of extract in the stages to the concentration of the extract at nth stage divided by the product of the flow of raffinate to the nth stage of concentration of the raffinate.
Sanfoundry Global Education & Learning Series – Bioseparation Processes.
To practice all areas of Bioseparation Processes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
