This set of Bioseparation Science Multiple Choice Questions & Answers (MCQs) focuses on “Adsorption Isotherms”.
1. The analysis of an adsorption process is based on the __________
a) equilibrium
b) identification
c) relationship
d) performance
View Answer
Explanation: The analysis of process of adsorption which is based on the identification of an equilibrium relationship between bound and free solute and performing a solute material balance.
2. What is an isotherm?
a) Equilibrium relationship
b) Identification
c) Relationship
d) Performance
View Answer
Explanation: The equilibrium relationship between the concentration of solute in the liquid phase and that on the surface of the adsorbent at a given condition is known as an isotherm.
3. The concentration of salt required for the adsorption of protein depends on ___________
a) type of adsorbent
b) type of adsorbate
c) type of salt
d) type of interaction
View Answer
Explanation: The concentration of salt required for the adsorption of protein depends on the type of salt used and when the sodium sulfate is used, a lower salt concentration is required than when using ammonium sulfate or sodium chloride.
4. The hydrophobic interaction is based on ___________
a) adsorption of water
b) adsorption of protein
c) adsorption of salts
d) adsorption of solute
View Answer
Explanation: The hydrophobic interaction is based on the adsorption of proteins which take place at very high anti-chaotropic concentrations of salt.
5. Which is the widely used support material for hydrophobic interactions?
a) Agarose
b) Agar
c) Sodium
d) Gelatin
View Answer
Explanation: The agarose is considered the most widely used support material for preparing hydrophobic interaction adsorbents. The hydrophobic patches on the surface of the adsorbent are formed by the process of grafting alkyl and aromatic hydrocarbon groups on the surface of agarose.
6. The solute concentration is expressed as ___________
a) number of valence electron
b) number of molecules
c) valency
d) number of moles
View Answer
Explanation: The concentration of solute is commonly expressed as the number of moles of solute per unit mass or the mass of the substrate or the volume of solvent or the volume of adsorbent.
7. What are the types of isotherms in the process of adsorption in bioseparation engineering?
a) Linear, langmuir
b) Freundlich, Langmuir, linear
c) Langmuir
d) Linear, freundlich
View Answer
Explanation: There are three types of isotherms in the process of adsorption in bioseparation engineering and they are linear, Freundlich and Langmuir. Most of the isotherms are linear when the solute concentration is very low. The Freundlich isotherm does not predict any saturation of the binding surface by the solute. The Langmuir isotherm is applicable when there is a strong specific interaction between the solute and the adsorbent.
8. Identify the marked isotherms on the given figure.
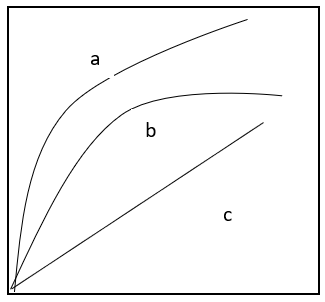
a) a-Freundlich, b-Langmuir, c-Linear
b) a-Langmuir, b-Freundlich, c-Linear
c) a-Linear, b-Langmuir, c-Freundlich
d) a-Langmuir, b-Linear, c-Freundlich
View Answer
Explanation: The various markings denotes that a stands for Freundlich isotherm, b is for Langmuir and c is for linear isotherm.
9. What is the equation for the linear isotherm?
a) CB = KCU
b) CB = K / CU
c) CB = K + CU
d) CB = K – CU
View Answer
Explanation: The equation for the linear isotherm is CB = KCU where, CB is solute bound per unit amount of adsorbent. is the linear equilibrium constant and CU is the unbound solute concentration in the solution.
10. Linear equilibrium is analogous to __________
a) refractive coefficient
b) partition coefficient
c) adsorbent
d) adsorbate
View Answer
Explanation: The K which is the linear equilibrium constant of the linear isotherm is analogous to the partition coefficient term used in extraction. Its unit depends on the units of the two concentration terms used in equation.
Sanfoundry Global Education & Learning Series – Bioseparation Science.
To practice all areas of Bioseparation Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
