This set of Bioseparation Processes Multiple Choice Questions & Answers (MCQs) focuses on “Precipitation”.
1. Which biological molecule can be purified using the process of precipitation?
a) Proteins and nucleic acid
b) Proteins
c) Nucleic acid
d) Carbohydrates
View Answer
Explanation: Protein precipitation is one of the most commonly used techniques in bioseparation. Once the cells are lysed, the products in the form of protein are to be purified and separated from the fermentation broth. The cytosolic proteins are easily soluble in water and their solubility depends on the pH as well as the ionic strength. The proteins are precipitated by adding salt in the fermentation broth.
2. The most common example of precipitation based bioseparation is _____________
a) Filtration
b) Cohn fractionation method
c) Sedimentation
d) Crystallization
View Answer
Explanation: Cohn fractionation method is the most commonly used method of precipitation in purification steps of bioseparation. It purifies plasma protein by extracting albumin from blood plasma. The process of precipitation is based on solubility difference of albumin and other plasma protein depending on the pH, concentration of ethanol, temperature, protein concentration and ionic strength.
3. How to separate the insoluble form of product which is obtained as precipitate from dissolved components?
a) Sedimentation
b) Filtration
c) Centrifugation
d) Evaporation
View Answer
Explanation: The precipitate can be separated from the fermentation broth through the centrifugation technique. The method of solid-liquid separation can be used to remove the precipitate from the dissolved components of the cell suspension.
4. What is the special type of precipitation process?
a) Membrane separation
b) Drying
c) Concentration
d) Crystallization
View Answer
Explanation: Crystallization is a special type of precipitation process which obtains the solid in a form of crystal. The formation of crystal is a slow process and is a highly controlled process and it requires the highly optimized conditions.
5. A normal precipitation process does not need to be precisely controlled.
a) True
b) False
View Answer
Explanation: Precipitation does not require highly optimized parameters or controlled process except the process of crystallization. It results in formation of solid material which us obtained in amorphous form in nature.
6. Which parameter is used for the solubility of proteins in aqueous solutions?
a) Pressure
b) Aeration
c) Temperature
d) Solubility
View Answer
Explanation: The solubility of proteins in aqueous solutions depends on the temperature and it can be proved by the profiles obtained by plotting solubility-temperature profiles of proteins. Solubility of one protein is more sensitive to the lowering of temperature than other protein. Separation can be carried out by lowering the temperature of the solution.
7. Which method is applicable for the precipitation of biological macromolecules?
a) Cooling
b) pH adjustment
c) solvent addition
d) Cooling, solvent addition, pH adjustment
View Answer
Explanation: The precipitation of biological molecules can be performed by adjusting the pH of the solution, cooling the solution, by adding the solvents in the solution, addition of chaotropic salts and addition of biospecific reagents.
8. Can cooling alone be used for precipitation.
a) True
b) False
View Answer
Explanation: Cooling alone cannot be used as a process of precipitation for biological product, however it can be used as an integral part of the precipitation process by involving organic solvents, partly due to synergistic solubility lowering the effects and partly due to the higher stability of the molecules at low temperature.
9. Which theory governs the process of Precipitation by using additives?
a) Thermodynamics
b) Kinetics
c) Rate order
d) 1st order reaction
View Answer
Explanation: The thermodynamics can govern the precipitation process when additives are used for precipitation of biological molecules. The thermodynamic equation is μppt = μliq where, μppt is the chemical potential of precipitated solute and μliq is the chemical potential of dissolved solute.
10. What is the composition of precipitate when a solute is being precipitated from its solution?
a) Solvent
b) Solute
c) Solution
d) Molecules
View Answer
Explanation: When a solute is being precipitated from its solution, the precipitate is mainly composed of solute and the chemical potential at any temperature remains constant while the chemical potential of dissolved solute depends on the concentration of the solution.
11. What is the equation for chemical potential of dissolved solute?
a) μppt = μliq
b) μppt = -μliq
c) μliq = μliq (0) + RT ln(x)
d) μliq = μliq (0) – RT ln(x)
View Answer
Explanation: The equation denotes the dependency of solute on the concentration and the equation is μliq = μliq (0) + RT ln(x) where, μliq (0) is the chemical potential of solute at standard reference state and x is the solute concentration.
12. What will increase the standard reference state of chemical potential?
a) Addition of salts
b) Addition of Solvent
c) Removal of solution
d) Addition of both salts and solvent
View Answer
Explanation: The salt and solvents are used as adding material for increasing the standard reference state of chemical potential. By adding these chemicals the concentration of solutes in the solution phase decreases in order to have the equal chemical potential of solute as well as the precipitate.
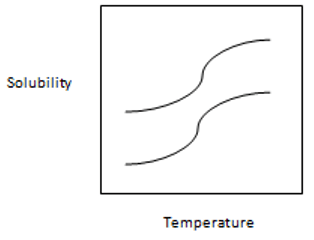
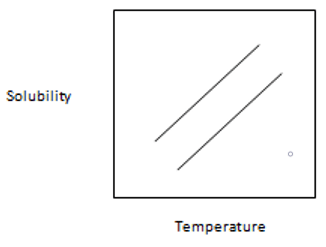
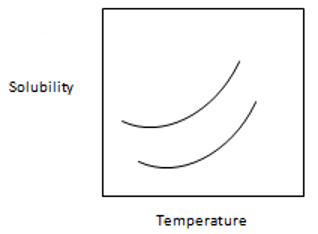
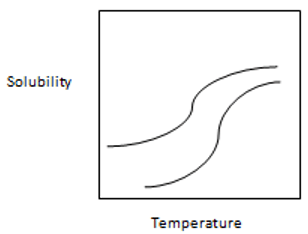
13. From the following graph, which one is best suitable for temperature-solubility profile of the two proteins A and B and it shows that solubility of A is more sensitive to lowering of temperature than B.
a)
b)
c)
d)
View Answer
Explanation: It shows the proper and clear picture of solubility of A and B in which separation of A and B are carried out by lowering the temperature of the solution to the point where A is precipitated more than B while B is more left in the solution. The solubility of A is more sensitive than B to the lowering of temperature.
More MCQs on Precipitation:
- Precipitation MCQ (Set 2)
- Precipitation MCQ (Set 3)
- Precipitation MCQ (Set 4)
- Precipitation MCQ (Set 5)
Sanfoundry Global Education & Learning Series – Bioseparation Processes.
To practice all areas of Bioseparation Processes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
