This set of Bioseparation Science Multiple Choice Questions & Answers (MCQs) focuses on “Batch Adsorption”.
1. In which adsorption process the feed solution and the adsorbent particles come together in contact until the equilibrium is achieved?
a) Continuous adsorption
b) Batch adsorption
c) Fed batch adsorption
d) Internal adsorption
View Answer
Explanation: The feed solution and the adsorbent particles are brought into contact until equilibrium binding is achieved in a batch adsorption process and it is used to adsorb solutes from liquid solutions when the quantities treated are small in amount.
2. The raffinate containing impurities are also known as ___
a) Spent solution
b) Feed
c) Supernatant
d) Pellet
View Answer
Explanation: A raffinate is a liquid stream that is left after the extraction with the immiscible liquid to remove solutes from the original liquid and hence the solution containing impurities are also termed as spent solution.
3. Which separation technique is applicable for the solid-liquid separation?
a) Adsorption
b) Sedimentation
c) Filtration
d) Electrophoresis
View Answer
Explanation: The spent solution also contains the impurities is then separated from the adsorbent by using an appropriate solid-liquid separation technique like filtration or process of centrifugation and the solid material is washed for the removal of impurities.
4. How can you elute the adsorbed solute from the adsorbent?
a) By adding solvent
b) By adding salt
c) By adding solute
d) By adding liquid
View Answer
Explanation: The adsorbed solute can then be eluted from the adsorbent by adding a liquid which favors desorption of the solute. It can be separated from the adsorbent by using appropriate solid- liquid separation technique of bioseparation.
5. With which isotherm, the analytical solution of batch adsorption is possible?
a) Freundlich
b) Langmuir
c) Linear
d) Both freundlich and linear
View Answer
Explanation: An analytical solution of batch adsorption is possible when the solute binding follows a linear adsorption isotherm because with Freundlich and Langmuir adsorption isotherms, graphical solutions are preferred.
6. What is the general form of equilibrium line?
a) CB = φCU
b) CB = φ – CU
c) CB = -φCU
d) CB = φ + CU
View Answer
Explanation: CB = φCU is considered the general form of the isotherm and is also termed as equilibrium line. The equilibrium line is given for the linear isotherm in terms of solute transport in the adsorption process.
7. What is the solute material balance equation of the batch adsorption?
a) CU0S – CB0A = CUS + CBA
b) CU0S + CB0A = CUS – CBA
c) CU0S + CB0A = CUS + CBA
d) CU0S + CB0A = – CUS + CBA
View Answer
Explanation: The equation for the solute material balance equation is CU0S + CB0A = CUS + CBA where, CU0 is initial solute concentration in the feed solution, CB0 is initial solute concentration in the adsorbent, S is quantity of feed solution, A is quantity of adsorbent.
8. What is the significance of the given diagram?
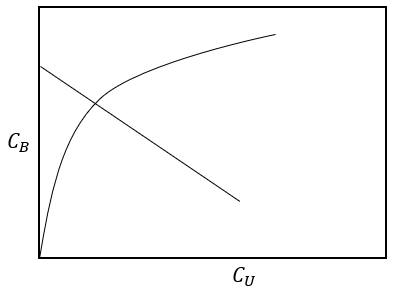
a) Process of continuous adsorption
b) Process of batch adsorption
c) Process of equilibrium intersection
d) Process of fed batch adsorption
View Answer
Explanation: The given diagram explains the point of intersection of the equilibrium line and the operating line gives the equilibrium concentrations i.e. CB and CU and the equation for it is CB = CB0 + \(\frac{S}{A}\)(CU0 – CU).
9. If hormone would be adsorbed in 100 ml of adsorbent were adsorbent used for a hormone which is being recovered from 30 l of a fluid, the adsorption follows linear isotherm and the concentration of the hormone in the fluid is 0.05 g/1. 90% of the hormone could be adsorbed in the batch mode by 30 ml of affinity adsorbent. Find the solute concentration in the adsorbent.
a) 1.379 \(\frac{g}{l}\)
b) 8.379 \(\frac{g}{l}\)
c) 10.379 \(\frac{g}{l}\)
d) 11.379 \(\frac{g}{l}\)
View Answer
Explanation: The total amount of hormone in feed is 30 * 0.05g = 1.5g and the amount of hormone bound to the adsorbent is 1.5 * 0.9 g is 1.35g and CB is \(\frac{\frac{1.35}{\frac{30}{1000}}g}{l}\) = \(\frac{45g}{l}\), if 90% of hormone in the feed is adsorbed CU = \(\frac{0.05 × 10}{100} \frac{g}{l}\) = 0.005 \(\frac{g}{l}\) and therefore, K = \(\frac{C_B}{C_U} = \frac{45}{0.005}\) = 9000. The equilibrium line is CB = 9000CU, when A is 100ml and the material balance is CB = \(\frac{30}{\frac{100}{1000}}\)(0.05 – CU) and CB is 11.379 \(\frac{g}{l}\) and CU is 0.00126 \(\frac{g}{l}\). Therefore, the solute concentration in the adsorbent is 11.379 \(\frac{g}{l}\).
10. In a freundlich isotherm for recovery of 20l of feed solution which best fits and how much adsorbent is required for 95% recovery of the antibiotic for the feed of 3.85 g/g water and the adsorption data for CU is 1.26 × 10-3 \(\frac{g}{l}\) and CB is 11.379 \(\frac{g}{l}\)?
a) 8.5 × 10-4g
b) 0.512g
c) .26 × 10-3g
d) 0.451g
View Answer
Explanation: The isotherm applicable is and the concentration of antibiotic in the feed is 3.85 × 10-5 g/g and S = 20 l = 20,000g so, A = \(\frac{20000}{11.379}\)(3.85 × 10-4 – 1.26 × 10-3) = 8.5 × 10-4g.
Sanfoundry Global Education & Learning Series – Bioseparation Science.
To practice all areas of Bioseparation Science, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
