This set of Bioseparation Processes Multiple Choice Questions & Answers (MCQs) focuses on “Batch Extraction”.
1. Which process of extraction in which the batch of feed solution is mixed with batch of extracting solvent?
a) Aqueous two phase extraction
b) Counter current extraction
c) Single stage extraction
d) Batch extraction
View Answer
Explanation: The batch extraction process is the one in which the batch of feed solution is mixed with the batch of extracting solvent in an extractor. It is the simplest and commonly used method of extraction which extracts solute from one immiscible layer in to other by shaking the two layers until equilibrium is attained after that the layers settle.
2. Which laboratories use the batch extraction process?
a) Chemical laboratories
b) Biological laboratories
c) Pharmaceutical laboratories
d) Biopharmaceutical laboratories
View Answer
Explanation: The chemical laboratories at small scale level use the batch extraction processes and it also used in the distribution ration in large scale. The extraction process in batches help the chemical industries to collect the products in sets and then is used for further settling and finally the products are separated.
3. Which apparatus is commonly used for performing the batch extraction at small scale?
a) Dryer
b) Filter
c) Separating funnel
d) Extractor
View Answer
Explanation: Separating funnel is used for performing batch extraction. It is used for separation of two liquids that do not dissolve each other. The pressure is build up in the funnel and it occurs due to a portion of the solvent which vaporizes when the funnel is shaken.
4. The rate at which the transfer of solute takes place from the feed to the extracting solvent depends on ____________
a) partition coefficient
b) mixing rate
c) settling point
d) concentration of solutes
View Answer
Explanation: The mixing rate is the parameter for the transfer of solutes from the feed to the extracting solvent. The partition coefficient is the parameter for solute distribution between the two phases. The settling point does not have much impact on the process of extraction. The concentration of solutes has indirect impact on the rate of transfer of solute.
5. What is the significance of the given diagram?
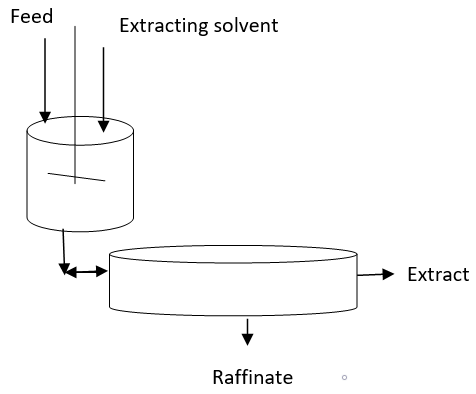
a) Basic principle of batch extraction using a mixer settler
b) Counter current extraction process
c) Aqueous two phase extraction
d) Mixer and extract fragmentation
View Answer
Explanation: The basic principle of batch extraction explains that the mixer unit is able to generate the high interfacial area and it must provide high solute mass transfer coefficient, can cause low entrainment of air bubbles. The settler unit must have a low aspect ratio and must allow easy coalescence and phase separation which allows easy collection of Raffinate as well as extract will be used as separate streams.
6. The antibiotic penicillin partitions favourably in an organic solvent from an aqueous fermentation media at ____________
a) basic condition
b) acidic condition
c) neutral condition
d) alkaline condition
View Answer
Explanation: The partition of penicillin is favoured at acidic conditions in organic solvents from an aqueous fermentation media. The neutral pH favours the partitioning from the organic phase to aqueous phase and the basic as well as alkaline pH does not affect the fermentation media of the antibiotic penicillin.
7. What is the significance of the diagram?
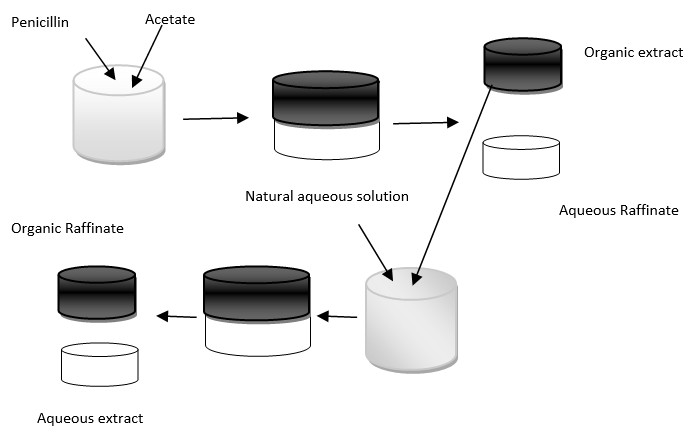
a) Batch extraction
b) Aqueous two phase extraction
c) Sequential reverse batch extraction
d) Counter current extraction
View Answer
Explanation: The diagram explains the sequential reverse batch extraction process in which the antibiotic can be purified by the sequential reversed batch extraction where the antibiotic is moved from aqueous to organic phase and back again. The shown sequence is usually a repeated few times in order to obtain highly pure antibiotic.
8. What is the concentration distribution in the extract and the Raffinate at equilibrium?
a) CE = CR
b) -CE = KCR
c) CE ≠ KCR
d) CE = KCR
View Answer
Explanation: If a batch of feed containing R amount of initial solvent and an initial solute concentration of CR0 is mixed with E amount of extracting solvent, the concentration distribution in the extract and the Raffinate at equilibrium is CE = KCR where, CE is solute concentration in extract and CR is the solute concentration in Raffinate.
9. Calculate the concentration of citric acid in both Extract and Raffinate, when the aqueous solution of citric acid is 100l and the contact was made with organic solvent where the solute concentration in extract was 100\(C_R^2\).
a) 0.270, 7.298
b) 7.298. 0.270
c) 0.50, 0.65
d) 0.230, 7.892
View Answer
Explanation: The mass balance between the first batch extractions was 100CR + 10CE = 100, the equilibrium relationship is CE = 100\(C_R^2\) and the CE = \(\frac{7.298g}{l}\). The concentration of citric acid in extract is 7.298 \(\frac{g}{l}\) and that of Raffinate is 0.270 \(\frac{g}{l}\).
10. Calculate the fraction of citric acid extracted when the aqueous solution of citric acid is 100l and the contact was made with organic solvent where the solute concentration in extract was 7.298 \(\frac{g}{l}\) and that of Raffinate is 0.270 \(\frac{g}{l}\).
a) 0.7298
b) 0.8970
c) 0.9870
d) 1.987
View Answer
Explanation: The fraction of citric acid extracted in the first batch extraction is P = \(\frac{EC_E}{RC_{R0}} = \frac{7.298 × 10}{100}\) = 0.7298.
11. Calculate the concentration of citric acid in the Raffinate and the extract phases of the second extraction when the aqueous solution of citric acid is 100l and the contact was made with organic solvent where the solute concentration in extract was 7.298 \(\frac{g}{l}\) and that of Raffinate is 0.270 \(\frac{g}{l}\) and the partition fraction is 0.7298.
a) 0.369 g/l, 13.609 g/l
b) 13.609 g/l, 13.609 g/l
c) 13.609 g/l, 0.369 g/l
d) 0.369g/l, 2.569g/l
View Answer
Explanation: The citric acid mass balance for the second batch extraction is (100 × 1) + (10 × 7.298) = 100CR + 10CE ∴ CE = 13.609 \(\frac{g}{l}\) and CR = 0.369 \(\frac{g}{l}\).
Sanfoundry Global Education & Learning Series – Bioseparation Processes.
To practice all areas of Bioseparation Processes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
