This set of Bioseparation Processes Multiple Choice Questions & Answers (MCQs) focuses on “Mechanism of Precipitate Formation”.
1. Which is the basic step involved in formation of precipitate?
a) Formation of tiny particles
b) Formation of big crystals
c) Formation of clusters
d) Formation of coagulants
View Answer
Explanation: The precipitate formation is a time dependent process and the basic step involved in it is the formation of tiny particles which is due to the association of macromolecules. These tiny particles collide with each other and increase in size.
2. How can you measure the process of precipitation?
a) Measuring the atmospheric pressure
b) Measuring the solubility
c) Measuring the temperature
d) Measuring the turbidity
View Answer
Explanation: Measurement of turbidity of the medium helps in measuring the process of precipitation which takes place as a function of time. The turbidity is the measure of the suspended solids in the suspension and it is analogous to the absorbance.
3. The greater the value of turbidity, the more precipitation took place and the suspended solid content is more.
a) False
b) True
View Answer
Explanation: The turbidity can be calculated by the measurement of the incident as well as transmitted light at a particular wavelength passing through a sample within the cuvette. The observed result states that if the measure of turbidity is more for a sample then the amount of suspended solid is also more proving that the turbidity as well as the precipitation is directly proportional to each other.
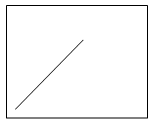
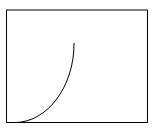
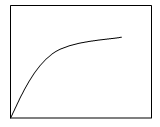
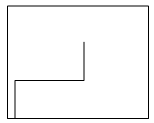
4. Which plot shows the relation between the turbidity with respect to time?
a)
b)
c)
d)
View Answer
Explanation: The precipitation process changes with change in turbidity and time. Therefore, the plot c shows the increase in the turbidity of the solution with increase in time and gradually it reaches equilibrium then the process of precipitation stops.
5. Which is considered to be the first step of precipitation?
a) Nucleation
b) Diffusion
c) Mixing
d) Convention
View Answer
Explanation: The first step of the formation of precipitate is homogeneous mixture of components including the macromolecules, solvent and the agent of precipitation. Mixing takes place over a finite amount of time, the time taken depends on the properties of the components of the mixture as well as on the mixing intensity, temperature.
6. Which process is involved in generation of particles of ultramicroscopic size of precipitation?
a) Nucleation
b) Mixing
c) Diffusion
d) Convention
View Answer
Explanation: Nucleation is the process of generation of particles of ultramicroscopic size and these particles of solute forms the supersaturated solution with respect to the solute. It is the process of determination of the new phase, in which the time required for the observer to wait before the initiation of new phase takes place with an organised structure.
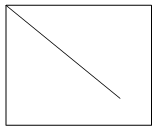
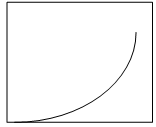
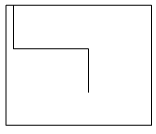
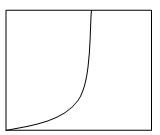
7. Which plot represents the nucleation rate as a function of degree of supersaturation?
a)
b)
c)
d)
View Answer
Explanation: In a supersaturated solution, the concentration of the solute is greater than the concentration at normal equilibrium. The difference between the actual concentration in solution and the concentration at equilibrium is referred to as supersaturation. The rate of nucleation increases to a high value at the supersaturation limit as mentioned in the plot d.
8. What will be the time required for mixing of the solutes in the solution in order to initiate the process of precipitation?
a) θ = \(\frac{l_e}{4D}\)
b) θ = –\(\frac{l_e^2}{4D}\)
c) θ = \(\frac{l_e^2}{4D}\)
d) θ = \(\frac{l_e^2}{D}\)
View Answer
Explanation: The time needed for the process of mixing is obtained by using θ = \(\frac{l_e^2}{4D}\) where, θ is the mixing time, D is diffusivity and le is the average eddy length. The average eddy length depends on the system volume, density and the viscisity of the medium.
9. Controlled supersaturation does not produce amorphous precipitates.
a) True
b) False
View Answer
Explanation: The controlled process of supersaturation produces an amorphous precipitates which can be easily filtered as well as centrifuged. It results in uneven mixing and the extent of supersaturation determines the nature of the precipitates.
10. Which process of bioseparation will be difficult for the supersaturated precipitate?
a) Membrane separation
b) Electrophoresis
c) Chromatography
d) Sedimentation
View Answer
Explanation: The very high degree of supersaturation leads to formation of gelatinous precipitates and these precipitates cannot be separated easily by the process of membrane separation or the process of filtration.
Sanfoundry Global Education & Learning Series – Bioseparation Processes.
To practice all areas of Bioseparation Processes, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
