This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Condensation Polymers”.
1. The _________ is utilized to synthesize polymers from monomers with 2 reactive functional groups by a step-wise reaction.
a) Addition polymer
b) Condensation polymer
c) Cross-linked addition
d) Addition monomer
View Answer
Explanation: The condensation mechanism is utilized to synthesize polymers from monomers with 2 or more for crosslinked polymer reactive function groups by a step-wise reaction.
2. Every step of the reaction b/w 2 monomers produces a simple molecule, often H2O, but sometimes NH3 or another molecule, as a main product.
a) True
b) False
View Answer
Explanation: These reactions are common, well known organic chemistry reactions. Each reaction step between 2 monomers produces a simple molecule, often H2O, but sometimes NH3 or another molecule, as a side product.
3. _________ are easily controlled synthetic polymer reactions, not like free radical reactions.
a) Precipitation reaction
b) Addition reaction
c) Radiation reaction
d) Condensation reaction
View Answer
Explanation:The most common reactions of synthetic polymers are esterification formation of ester linkages and amidation formation of amide linkages. Condensation reactions are easily controlled reactions, unlike free radical reactions.
4. In the case of _________ it is possible to utilize a single compound to make the polymer, and that’s H2N(CH2)5COOH.
a) Nylon6, 6
b) Nylon2, 2
c) Methamphetamine
d) Desoxyn
View Answer
Explanation: In the case of nylon 6,6 (6 is the number of carbon atoms in each of the two monomers if a di-carboxylic acid and di-amine were utilized to form it) it is possible to utilize a single compound to make the polymer, and that’s H2N(CH2)5COOH.
5. After a short time essentially all of the monomers will be gone and oligomers will exist in the condensation reaction.
a) True
b) False
View Answer
Explanation: The monomers will react together initially and small polymers (oligomers) will react later. This means that after a short time essentially all of the monomers will be gone and oligomers will exist.
6. Oligomers react to form short chain polymers, then the short chained polymers react to form moderately long chain polymers. The number _________ can be predicted as a function of the extent of reaction.
a) Average DP
b) John DP
c) Pascal DP
d) Rankine DP
View Answer
Explanation: The number average degree of polymerisation can be used to predicted the function of the extent of reaction. These oligomers will then react to form short chain polymers, then the short chained polymers will react to form moderately long chain polymers.
7. What is the name of the compound?
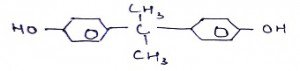
a) Ethylene glycol
b) Diphenyl propane
c) Hexaneamide
d) Nylon(6, 6)
View Answer
Explanation: 1,3-Diphenyl-1,3-propanedione (dibenzoylmethane, D.B.M.) is an aromatic 1,3-diketone derivative of acetylacetone, where both methyl groups in acetylacetone have been substituted by phenyl groups. It’s a white solid which melts at 77−78 °Celcius.
8. What is the name of the compound?
HOCH2CH2OH
a) Ethylene glycol
b) Diphenyl propane
c) Hexaneamide
d) Nylon6, 6
View Answer
Explanation: Ethylene glycol (I.U.P.A.C. name: ethane-1,2-diol) is an organic compound primarily used as a raw material in the manufacture of polyester fibers and fabric industry, and polyethylene terephthalate resins (P.E.T.) utilized in bottling.
9. What is the name of the compound?
-NH(CH2)6NH-CO(CH2)4CO-
a) Ethylene glycol
b) Diphenyl propane
c) Hexaneamide
d) Nylon(6, 6)
View Answer
Explanation: Nylon 66 (also known as nylon 6-6, nylon 6/6 or nylon 6,6) is made of 2 monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid, which produces nylon 66, its name.
10. What is the name of the product?
H2N(CH2)6NH2 + HOOC-(CH2)4-COOH andrarr; ___________
a) Ethylene glycol
b) Diphenyl propane
c) Hexaneamide
d) Nylon6,6
View Answer
Explanation: Nylon 66 (aka nylon 6-6, nylon 6/6 or nylon 6,6) is a type of polyamide or nylon. Nylons come in many types, and the two most common for textile and plastics industries are nylon 6 and nylon 6,6.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Practice Chemical Engineering MCQs
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
