This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Surfactants”.
1. _________are surface-active agents that aggregate near or have a strong effect on modifying the interface b/w 2 materials.
a) Carboxylate
b) Tween
c) Ampholytic elements
d) Surfactants
View Answer
Explanation: This occurs because of their dual nature: hydrophobic and hydrophilic. It’s a substance which tends to decrement the surface tension of a liquid in which it’s dissolved in it.
2. The hydrophilic moiety may be anionic, cationic, ampholytic, or non-ionic not depending on the type of charge(s) carried, if any.
a) True
b) False
View Answer
Explanation: The hydrophilic moiety may be anionic (carboxylate, sulfate, or phosphate), cationic ,ampholytic, or nonionic (Span®, Tween®, Triton®) depending on the type of charge(s) carried, if any.
3. Anionic surfactants are generally less expensive than cationic ones, though the latter sometimes have biocidal action.
a) True
b) False
View Answer
Explanation: In the anionic surfactants, the carboxylate group in soap is replaced by a sulfonate or as the hydrophilic component. The corresponding Ca and Mg salts are more soluble in H2O than the Ca and Mg salts of carboxylic acids.
4. The Span materials tend to be less water soluble than the _________materials. The same material could be a detergent, and a wetting agent.
a) Carboxylate
b) Tween
c) Ampholytic elements
d) Surfactants
View Answer
Explanation: The Span materials tend to be less water soluble than the Tween materials. Surfactants are often known acc. to their use. The same material could be an emulsifier, or a dispersant.
5. The _________ of surfactants depends the most crucial factor that is strong water—water interaction, the hydrophobic effect.
a) CMC
b) Micelle
c) Polarity
d) Non-polarity
View Answer
Explanation: This was discovered by Shaw in 1980. The nonpolar—nonpolar interaction in the centre of the micelle doesn’t provide the thermodynamic energy required for micelle formation. .
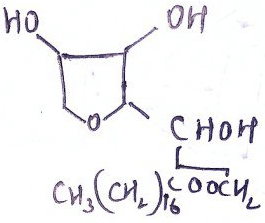
6. What is the type of hexahydric alcohol?
a) Tween
b) Span
c) Triton
d) Multi-cyclic
View Answer
Explanation: A member of the mannitol-sorbitol-dulcitol sugar group; isomer of C6H8(OH)6.
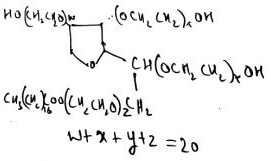
7. What is the type of hexahydric alcohol?
a) Tween
b) Span
c) Triton
d) Multi-cyclic
View Answer
Explanation: This comes under the category of mannitol-sorbitol-dulcitol sugar group; isomer of C6H8(OH)6.
8. What is the type of hexahydric alcohol?

a) Tween
b) Span
c) Triton
d) Multi-cyclic
View Answer
Explanation: A member of the mannitol-sorbitol-dulcitol sugar group; isomer of C6H8(OH)6.
9. What is the type of hexahydric alcohol?
CH3C(CH3)2CH2C(CH3)2
n = 9 for octoxynol
a) Tween
b) Span
c) Triton
d) Multi-cyclic
View Answer
Explanation: This belongs to the mannitol-sorbitol-dulcitol sugar group; isomer of C6H8(OH)6.
10. Many surfactants that form micelles have a solubility below their C.M.C. Thus at a certain temperature, known as the _________ point, the solubility increases dramatically since the micelles are quite soluble.
a) Mars
b) Kraft
c) Kraffin
d) Curtis
View Answer
Explanation: This belongs to the mannitol-sorbitol-dulcitol sugar group; isomer of C6H8(OH)6.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Apply for Chemical Engineering Internship
- Practice Chemical Engineering MCQs
