This set of Pulp and Paper Multiple Choice Questions and Answers (MCQs) focuses on “Addition Polymers”.
1. There’s only 1 category for types of polymerization reactions utilized to form polymers: condensation mechanism to form condensation polymers.
a) True
b) False
View Answer
Explanation: There are 2 categories for types of polymerization reactions used to form polymers: condensation and addition mechanisms to form condensation and addition polymers.
2. The _________ is utilized to make polymers from monomers with ring structures or double bonds by a chain reaction.
a) Condensation mechanism
b) Addition mechanism
c) Crosslink mechanism
d) Parallel mechanism
View Answer
Explanation: The addition mechanism is utilized to make polymers from monomers with ring structures or double bonds by a chain reaction. The “extra” bond of the monomer is utilized to form the bond b/w monomers.
3. Many molecules are lost during polymerization; that is, there is change in the molecular wt. of the monomer incorporated into the polymer.
a) True
b) False
View Answer
Explanation: No molecules are lost during polymerization. There is no change in the molecular weight of the monomer incorporated into the polymer.
4. _________ are usually formed by free radical reactions; however, anionic and cationic mechanisms may also be utilized, but require special solvents and reaction conditions.
a) Monomers
b) Polymers
c) Plastic
d) Carbon sox
View Answer
Explanation: Free radical reactions are started using initiators. These polymers are usually formed by free radical reactions; however, anionic and cationic mechanisms may also be utilized, but require special solvents and reaction conditions.
5. _________ are compounds that form free radicals, such as H2O2, to initiate the reaction. The free radical’s always carried by the terminal C atom b/w propagation steps.
a) Procrastinators
b) Inhibitors
c) Initiators
d) Monomers
View Answer
Explanation: Initiators are compounds that form free radicals, like H2O2, to initiate the reaction. The free radical is always carried by the terminal C atom b/w propagation steps. It could also be carried out with high energy radiation, photolysis, or thermal energy.
6. _________ almost invariably add head to head, -CH2CHR-CH2CHR-, as opposed to head to tail, which is -CHjCHR-CHRCHj-.
a) Procrastinators
b) Inhibitors
c) Initiators
d) Monomers
View Answer
Explanation: Head to head addition could form three types of polymers which differ in rotation around a C-C single bond. In atactic polymers the R groups are randomly oriented around the longitudinal axis of the polymer. Monomers almost invariably add head to head, -CH2CHR-CH2CHR-, as opposed to head to tail, which is -CHjCHR-CHR-CHj-.
7. What is the name of the compound?
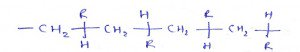
a) Syndiotactic
b) Isotactic
c) Polytactic
d) Butyl tactic
View Answer
Explanation: Syndiotactic polymers are referred to as stereoregular—that is, polymers which have an ordered arrangement of pendant groups along the chain. A polymer with a random orientation of groups is observed to be atactic.
8. What is the name of the compound?
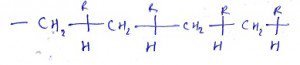
a) Syndiotactic
b) Isotactic
c) Polytactic
d) Butyl tactic
View Answer
Explanation: Syndiotactic polymers are usually high-strength materials because the uniform structure which leads to close packing of the polymer chains and a high degree of crystallinity.
9. What is the name of the process?
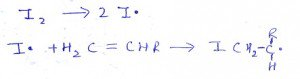
a) Initiator
b) Propagation
c) Termination
d) Chain transfer
View Answer
Explanation: It’s the initiation point of the reaction where I2 gas forms 2 free radicals for propagation process.
10. What is the name of the process?
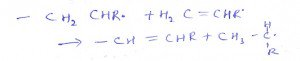
a) Initiator
b) Propagation
c) Termination
d) Chain transfer
View Answer
Explanation: This is the step where the propagation is carried out in a particular way by sharing electrons by the radical structure.
Sanfoundry Global Education & Learning Series – Pulp and Paper.
To practice all areas of Pulp and Paper, here is complete set of 1000+ Multiple Choice Questions and Answers.
If you find a mistake in question / option / answer, kindly take a screenshot and email to [email protected]
- Check Chemical Engineering Books
- Practice Chemical Engineering MCQs
- Apply for Chemical Engineering Internship
